Korean J Radiol.
2017 Feb;18(1):152-161. 10.3348/kjr.2017.18.1.152.
Added Value of Contrast-Enhanced Ultrasound on Biopsies of Focal Hepatic Lesions Invisible on Fusion Imaging Guidance
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea. leeminwoo0@gmail.com
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea.
- KMID: 2468129
- DOI: http://doi.org/10.3348/kjr.2017.18.1.152
Abstract
OBJECTIVE
To assess whether contrast-enhanced ultrasonography (CEUS) with Sonazoid can improve the lesion conspicuity and feasibility of percutaneous biopsies for focal hepatic lesions invisible on fusion imaging of real-time ultrasonography (US) with computed tomography/magnetic resonance images, and evaluate its impact on clinical decision making.
MATERIALS AND METHODS
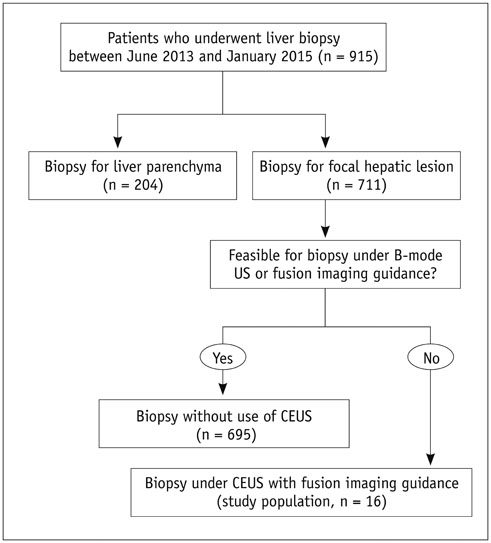
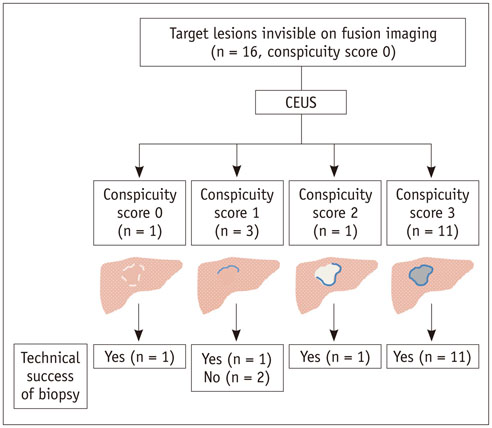
The Institutional Review Board approved this retrospective study. Between June 2013 and January 2015, 711 US-guided percutaneous biopsies were performed for focal hepatic lesions. Biopsies were performed using CEUS for guidance if lesions were invisible on fusion imaging. We retrospectively evaluated the number of target lesions initially invisible on fusion imaging that became visible after applying CEUS, using a 4-point scale. Technical success rates of biopsies were evaluated based on histopathological results. In addition, the occurrence of changes in clinical decision making was assessed.
RESULTS
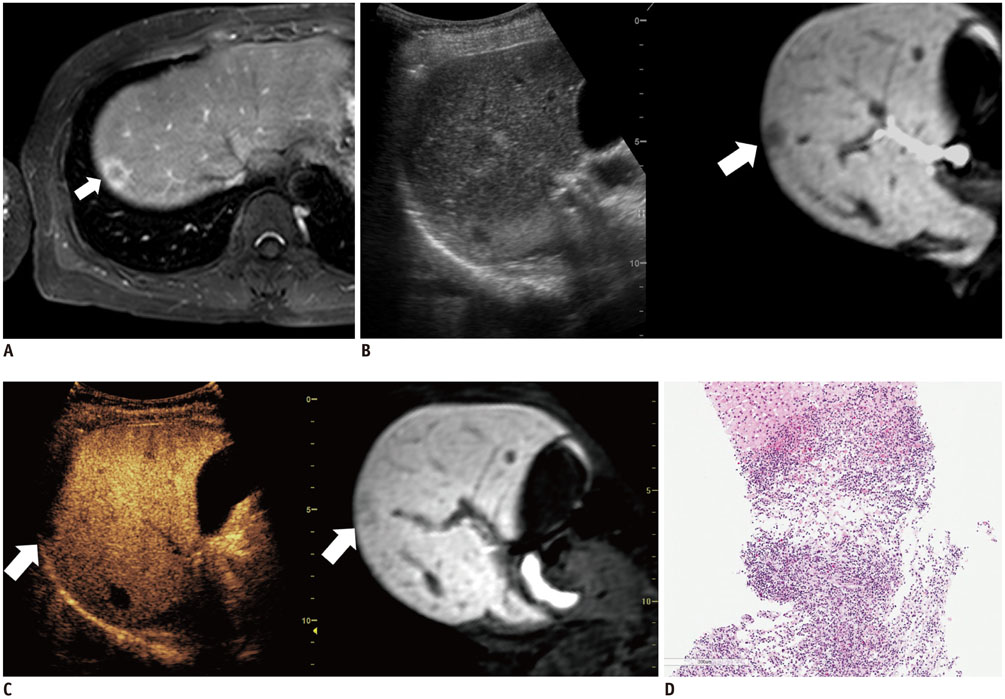
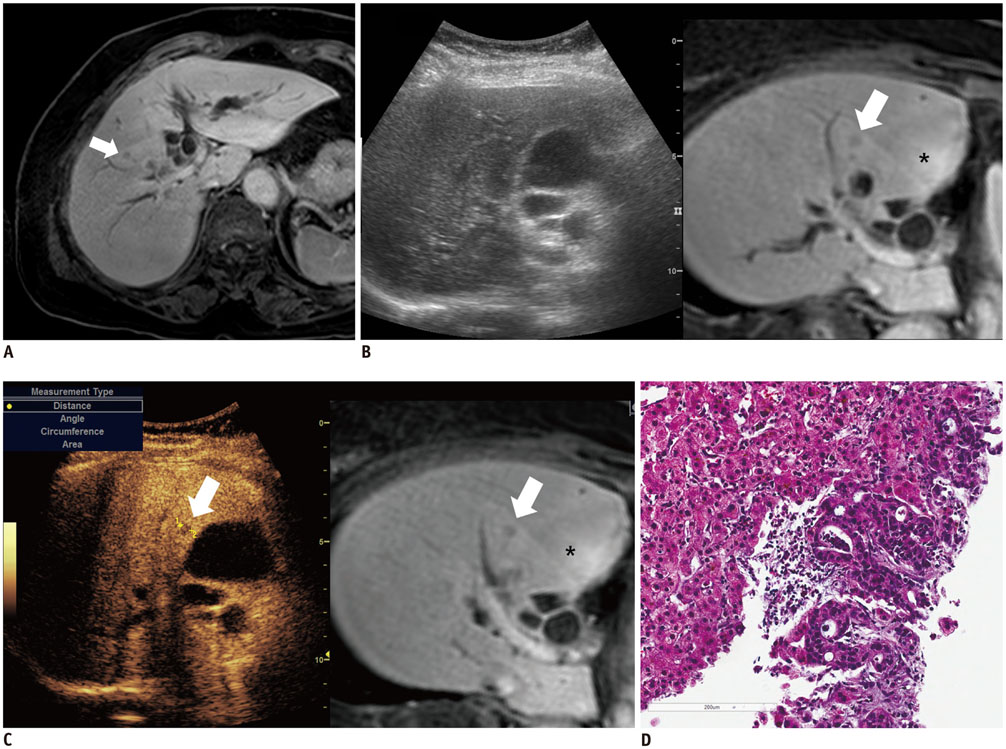
Among 711 patients, 16 patients (2.3%) were included in the study. The median size of target lesions was 1.1 cm (range, 0.5-1.9 cm) in pre-procedural imaging. After CEUS, 15 of 16 (93.8%) focal hepatic lesions were visualized. The conspicuity score was significantly increased after adding CEUS, as compared to that on fusion imaging (p < 0.001). The technical success rate of biopsy was 87.6% (14/16). After biopsy, there were changes in clinical decision making for 11 of 16 patients (68.8%).
CONCLUSION
The addition of CEUS could improve the conspicuity of focal hepatic lesions invisible on fusion imaging. This dual guidance using CEUS and fusion imaging may affect patient management via changes in clinical decision-making.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Clinical Decision-Making
Contrast Media/chemistry
Endoscopic Ultrasound-Guided Fine Needle Aspiration
Female
Ferric Compounds/chemistry
Humans
Iron/chemistry
Liver Diseases/*diagnosis/diagnostic imaging/pathology
Male
Middle Aged
Oxides/chemistry
*Positron Emission Tomography Computed Tomography
Retrospective Studies
Young Adult
Contrast Media
Ferric Compounds
Oxides
Iron
Figure
Cited by 1 articles
-
Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010; 45:133–141.2. Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008; 28:1983–1998.3. Francis IR, Cohan RH, McNulty NJ, Platt JF, Korobkin M, Gebremariam A, et al. Multidetector CT of the liver and hepatic neoplasms: effect of multiphasic imaging on tumor conspicuity and vascular enhancement. AJR Am J Roentgenol. 2003; 180:1217–1224.4. Devi CR. Enlightened oncologists can provide quality cancer care at reduced costs. J Surg Oncol. 2014; 110:643–644.5. Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014; 33:227–239.6. Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005; 242:158–171.7. Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003; 226:533–542.8. Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002; 224:748–756.9. Jung EM, Friedrich C, Hoffstetter P, Dendl LM, Klebl F, Agha A, et al. Volume navigation with contrast enhanced ultrasound and image fusion for percutaneous interventions: first results. PLoS One. 2012; 7:e33956.10. Park HS, Kim YJ, Yu MH, Jung SI, Jeon HJ. Real-time contrast-enhanced sonographically guided biopsy or radiofrequency ablation of focal liver lesions using perflurobutane microbubbles (sonazoid): value of Kupffer-phase imaging. J Ultrasound Med. 2015; 34:411–421.11. Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1-3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013; 24:958–965.12. Lim S, Lee MW, Rhim H, Cha DI, Kang TW, Min JH, et al. Mistargeting after fusion imaging-guided percutaneous radiofrequency ablation of hepatocellular carcinomas. J Vasc Interv Radiol. 2014; 25:307–314.13. Min JH, Lim HK, Lim S, Kang TW, Song KD, Choi SY, et al. Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol. 2014; 20:61–70.14. Minami T, Minami Y, Chishina H, Arizumi T, Takita M, Kitai S, et al. Combination guidance of contrast-enhanced US and fusion imaging in radiofrequency ablation for hepatocellular carcinoma with poor conspicuity on contrast-enhanced US/fusion imaging. Oncology. 2014; 87:Suppl 1. 55–62.15. Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015; 4:176–187.16. Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2015; 34:3–18.17. Nishigaki Y, Hayashi H, Tomita E, Suzuki Y, Watanabe N, Watanabe S, et al. Usefulness of contrast-enhanced ultrasonography using Sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2015; 45:432–440.18. Moriyasu F, Itoh K. Efficacy of perflubutane microbubble-enhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2009; 193:86–95.19. Park HJ, Lee MW, Lee MH, Hwang J, Kang TW, Lim S, et al. Fusion imaging-guided percutaneous biopsy of focal hepatic lesions with poor conspicuity on conventional sonography. J Ultrasound Med. 2013; 32:1557–1564.20. Yoon SH, Lee KH, Kim SY, Kim YH, Kim JH, Lee SH, et al. Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol. 2010; 20:2047–2056.21. Goto E, Masuzaki R, Tateishi R, Kondo Y, Imamura J, Goto T, et al. Value of post-vascular phase (Kupffer imaging) by contrast-enhanced ultrasonography using Sonazoid in the detection of hepatocellular carcinoma. J Gastroenterol. 2012; 47:477–485.22. Jang JY, Kim MY, Jeong SW, Kim TY, Kim SU, Lee SH, et al. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin Mol Hepatol. 2013; 19:1–16.23. Lee MW, Rhim H, Cha DI, Kim YJ, Choi D, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012; 198:1438–1444.24. Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016; 280:300–312.25. Mishima M, Toh U, Iwakuma N, Takenaka M, Furukawa M, Akagi Y. Evaluation of contrast Sonazoid-enhanced ultrasonography for the detection of hepatic metastases in breast cancer. Breast Cancer. 2016; 23:231–241.26. Tanaka M, Nakashima O, Wada Y, Kage M, Kojiro M. Pathomorphological study of Kupffer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology. 1996; 24:807–812.27. Watanabe R, Matsumura M, Munemasa T, Fujimaki M, Suematsu M. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol. 2007; 42:643–651.28. Elsayes KM, Ellis JH, Elkhouly T, Ream JM, Bowerson M, Khan A, et al. Diagnostic yield of percutaneous image-guided tissue biopsy of focal hepatic lesions in cancer patients: ten percent are not metastases from the primary malignancy. Cancer. 2011; 117:4041–4048.29. Alagoz O, Chhatwal J, Burnside ES. Optimal policies for reducing unnecessary follow-up mammography exams in breast cancer diagnosis. Decis Anal. 2013; 10:200–224.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention
- Contrast-enhanced ultrasonography of the liver using SonoVue
- New Ultrasonographic Techniques to Differentiate Hepatic Masses: Contrast-Enhanced Ultrasound and Super-Resolution Ultrasound
- Multislice computed tomography/contrast-enhanced ultrasound image fusion as a tool for evaluating unclear renal cysts
- Medical imaging of prostate cancer