Korean J Radiol.
2017 Feb;18(1):84-93. 10.3348/kjr.2017.18.1.84.
Update on Gastrointestinal Stromal Tumors for Radiologists
- Affiliations
-
- 1Department of Imaging, Dana-Farber Cancer Institute, Boston, MA 02215, USA. stirumani@partners.org
- 2Department of Radiology, Brigham and Women's Hospital, Boston, MA 02115, USA.
- 3Department of Radiology, Tata Memorial Centre, Mumbai 400012, India.
- 4Department of Radiology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
- KMID: 2468124
- DOI: http://doi.org/10.3348/kjr.2017.18.1.84
Abstract
- The management of gastrointestinal stromal tumors (GISTs) has evolved significantly in the last two decades due to better understanding of their biologic behavior as well as development of molecular targeted therapies. GISTs with exon 11 mutation respond to imatinib whereas GISTs with exon 9 or succinate dehydrogenase subunit mutations do not. Risk stratification models have enabled stratifying GISTs according to risk of recurrence and choosing patients who may benefit from adjuvant therapy. Assessing response to targeted therapies in GIST using conventional response criteria has several potential pitfalls leading to search for alternate response criteria based on changes in tumor attenuation, volume, metabolic and functional parameters. Surveillance of patients with GIST in the adjuvant setting is important for timely detection of recurrences.
MeSH Terms
-
Antineoplastic Agents/therapeutic use
Benzamides/therapeutic use
Chemotherapy, Adjuvant
Combined Modality Therapy
Exons
Gastrointestinal Neoplasms/*diagnostic imaging/drug therapy/genetics
Gastrointestinal Stromal Tumors/*diagnostic imaging/drug therapy/genetics
Humans
Imatinib Mesylate/therapeutic use
Mutation
Neoplasm Recurrence, Local
Pyrimidines/therapeutic use
Succinate Dehydrogenase/genetics
Tomography, X-Ray Computed
Antineoplastic Agents
Benzamides
Pyrimidines
Imatinib Mesylate
Succinate Dehydrogenase
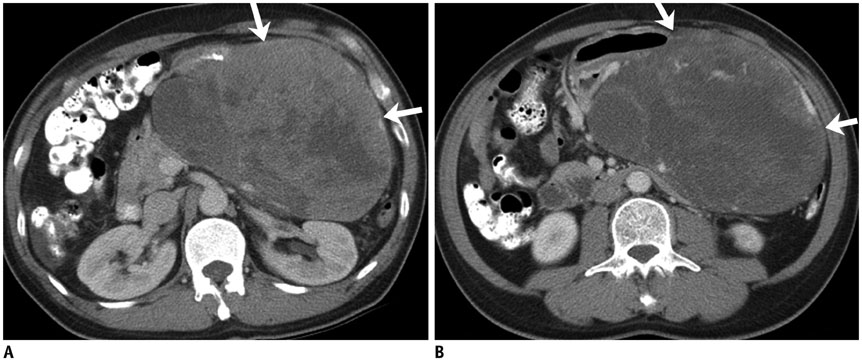
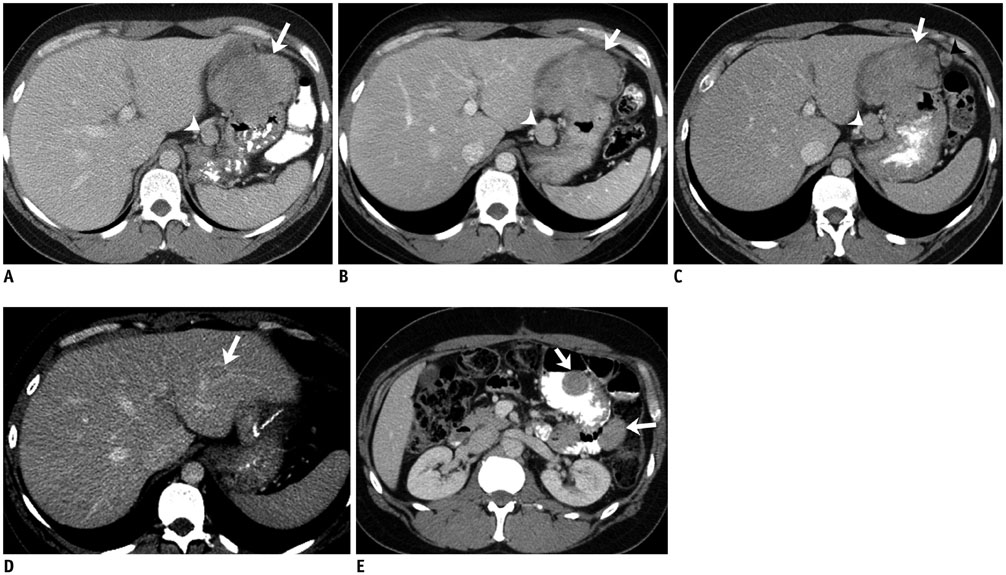
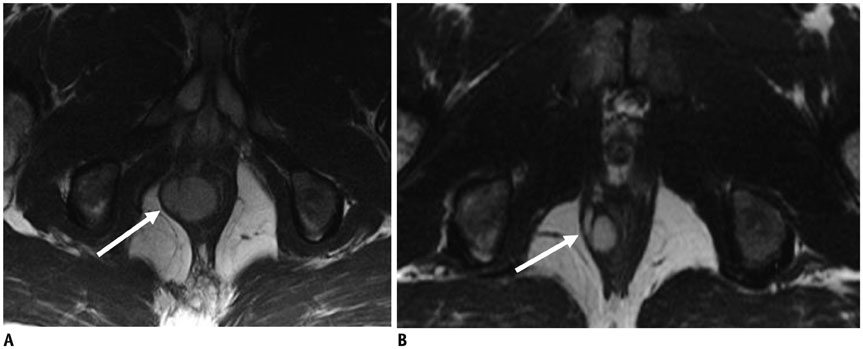
Figure
Reference
-
1. Liegl B, Hornick JL, Lazar AJ. Contemporary pathology of gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009; 23:49–68. vii–viii.2. Call J, Walentas CD, Eickhoff JC, Scherzer N. Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry. BMC Cancer. 2012; 12:90.3. Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002; 33:466–477.4. Tirumani SH, Jagannathan JP, Krajewski KM, Shinagare AB, Jacene H, Ramaiya NH. Imatinib and beyond in gastrointestinal stromal tumors: a radiologist’s perspective. AJR Am J Roentgenol. 2013; 201:801–810.5. Hornick JL, Fletcher CD. The significance of KIT (CD117) in gastrointestinal stromal tumors. Int J Surg Pathol. 2004; 12:93–97.6. Marrari A, Wagner AJ, Hornick JL. Predictors of response to targeted therapies for gastrointestinal stromal tumors. Arch Pathol Lab Med. 2012; 136:483–489.7. Gronchi A. Risk stratification models and mutational analysis: keys to optimising adjuvant therapy in patients with gastrointestinal stromal tumour. Eur J Cancer. 2013; 49:884–892.8. Wardelmann E, Büttner R, Merkelbach-Bruse S, Schildhaus HU. Mutation analysis of gastrointestinal stromal tumors: increasing significance for risk assessment and effective targeted therapy. Virchows Arch. 2007; 451:743–749.9. Maleddu A, Pantaleo MA, Nannini M, Di Battista M, Saponara M, Lolli C, et al. Mechanisms of secondary resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumours (review). Oncol Rep. 2009; 21:1359–1366.10. O’Regan KN, Shinagare AB, Saboo SS, Ramaiya NH, Jagannathan JP, Tirumani SH. Gastrointestinal stromal tumors (GIST): lesser known facts. Clin Imaging. 2013; 37:821–829.11. Tirumani SH, Tirumani H, Jagannathan JP, Shinagare AB, Hornick JL, George S, et al. MDCT features of succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumours. Br J Radiol. 2014; 87:20140476.12. Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003; 9:3329–3337.13. Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011; 35:1712–1721.14. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010; 28:1247–1253.15. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003; 21:4342–4349.16. Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008; 26:5360–5367.17. Tirumani SH, Jagannathan JP, Hornick JL, Ramaiya NH. Resistance to treatment in gastrointestinal stromal tumours: what radiologists should know. Clin Radiol. 2013; 68:e429–e437.18. Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006; 24:4764–4774.19. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–465.20. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23:70–83.21. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419.22. Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011; 37:890–896.23. Goh BK, Chow PK, Yap WM, Kesavan SM, Song IC, Paul PG, et al. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol. 2008; 15:2153–2163.24. Huang HY, Li CF, Huang WW, Hu TH, Lin CN, Uen YH, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007; 141:748–756.25. Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009; 10:1045–1052.26. Chok AY, Goh BK, Koh YX, Lye WK, Allen JC Jr, Quek R, et al. Validation of the MSKCC gastrointestinal stromal tumor nomogram and comparison with other prognostication systems: single-institution experience with 289 patients. Ann Surg Oncol. 2015; 22:3597–3605.27. Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012; 13:265–274.28. O’Neill AC, Shinagare AB, Kurra V, Tirumani SH, Jagannathan JP, Baheti AD, et al. Assessment of metastatic risk of gastric GIST based on treatment-naïve CT features. Eur J Surg Oncol. 2016; 42:1222–1228.29. Tirumani SH, Shinagare AB, Jagannathan JP, Krajewski KM, Ramaiya NH, Raut CP. Radiologic assessment of earliest, best, and plateau response of gastrointestinal stromal tumors to neoadjuvant imatinib prior to successful surgical resection. Eur J Surg Oncol. 2014; 40:420–428.30. NCCN Clinical Practice Guidelines in Oncology. Soft Tissue Sarcoma Version 2. NCCN;2016. Accessed March 31, 2016. Web site. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.31. Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012; 307:1265–1272.32. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480.33. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006; 368:1329–1338.34. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381:295–302.35. Kang YK, Ryu MH, Yoo C, Ryoo BY, Kim HJ, Lee JJ, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013; 14:1175–1182.36. Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004; 183:1619–1628.37. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007; 25:1753–1759.38. Rezai P, Pisaneschi MJ, Feng C, Yaghmai V. A radiologist’s guide to treatment response criteria in oncologic imaging: anatomic imaging biomarkers. AJR Am J Roentgenol. 2013; 201:237–245.39. Tirumani SH, Shinagare AB, O’Neill AC, Nishino M, Rosenthal MH, Ramaiya NH. Accuracy and feasibility of estimated tumour volumetry in primary gastric gastrointestinal stromal tumours: validation using semiautomated technique in 127 patients. Eur Radiol. 2016; 26:286–295.40. Schiavon G, Ruggiero A, Bekers DJ, Barry PA, Sleijfer S, Kloth J, et al. The effect of baseline morphology and its change during treatment on the accuracy of Response Evaluation Criteria in Solid Tumours in assessment of liver metastases. Eur J Cancer. 2014; 50:972–980.41. Schiavon G, Ruggiero A, Schöffski P, van der Holt B, Bekers DJ, Eechoute K, et al. Tumor volume as an alternative response measurement for imatinib treated GIST patients. PLoS One. 2012; 7:e48372.42. Schramm N, Englhart E, Schlemmer M, Hittinger M, Übleis C, Becker CR, et al. Tumor response and clinical outcome in metastatic gastrointestinal stromal tumors under sunitinib therapy: comparison of RECIST, Choi and volumetric criteria. Eur J Radiol. 2013; 82:951–958.43. Shinagare AB, Barysauskas CM, Braschi-Amirfarzan M, O’Neill AC, Catalano PJ, George S, et al. Comparison of performance of various tumor response criteria in assessment of sunitinib activity in advanced gastrointestinal stromal tumors. Clin Imaging. 2016; 40:880–884.44. Shinagare AB, Jagannathan JP, Kurra V, Urban T, Manola J, Choy E, et al. Comparison of performance of various tumour response criteria in assessment of regorafenib activity in advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib. Eur J Cancer. 2014; 50:981–986.45. Van den Abbeele AD, Badawi RD, Tetrault RJ, Cliche JP, Manola J, Spangler T, et al. FDG-PET as a surrogate marker for response to Gleevec (TM) (imatinib mesylate) in patients with advanced gastrointestinal stromal tumors (GIST). J Nucl Med. 2003; 44:24–25.46. Van den Abbeele AD for the GIST Collaborative PET Study Group. F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST) [abstract]. Proc Am Soc Clin Oncol. 2001; 20:362a.47. Van den Abbeele AD. The lessons of GIST--PET and PET/CT: a new paradigm for imaging. Oncologist. 2008; 13:Suppl 2. 8–13.48. ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23:Suppl 7. vii49–vii55.49. Meyer M, Hohenberger P, Apfaltrer P, Henzler T, Dinter DJ, Schoenberg SO, et al. CT-based response assessment of advanced gastrointestinal stromal tumor: dual energy CT provides a more predictive imaging biomarker of clinical benefit than RECIST or Choi criteria. Eur J Radiol. 2013; 82:923–928.50. Betz M, Kopp HG, Spira D, Claussen CD, Horger M. The benefit of using CT-perfusion imaging for reliable response monitoring in patients with gastrointestinal stromal tumor (GIST) undergoing treatment with novel targeted agents. Acta Radiol. 2013; 54:711–721.51. Shankar S, vanSonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005; 235:892–898.52. Joensuu H, Reichardt P, Eriksson M, Sundby Hall K, Vehtari A. Gastrointestinal stromal tumor: a method for optimizing the timing of CT scans in the follow-up of cancer patients. Radiology. 2014; 271:96–103.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of huge Gastrointestinal stromal tumor masquerading as an ovarian malignancy
- A Case of Massive Bleeding from Jejunal Stromal Tumor Diagnosed by Intraoperative Enteroscopy: A Case of Jejunal Stromal Tumor Bleeding
- Clinical Relevance of the Location of Gastric Gastrointestinal Stromal Tumors
- Multiple Gastrointestinal Stromal Tumors of the Small Intestine
- Primary Extragastrointestinal Stromal Tumor of Retroperitoneum: Poor Response to Tyrosine Kinase Inhibitor