Nat Prod Sci.
2019 Dec;25(4):317-325. 10.20307/nps.2019.25.4.317.
Anti-inflammatory and Immunosuppressive Effects of Panax notoginseng
- Affiliations
-
- 1College of Pharmacy, Drug Research and Development Center, Daegu Catholic University, Gyeongbuk 38430, Republic of Korea. bsmin@cu.ac.kr
- KMID: 2468065
- DOI: http://doi.org/10.20307/nps.2019.25.4.317
Abstract
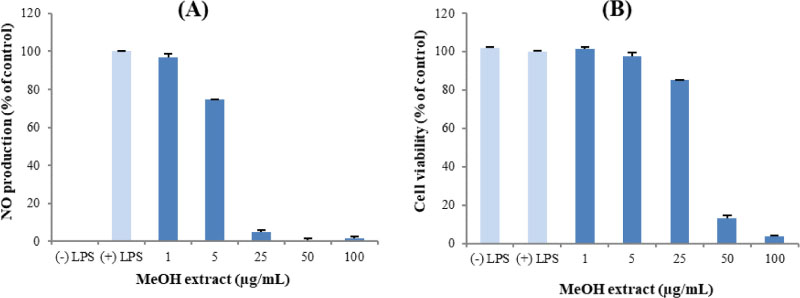
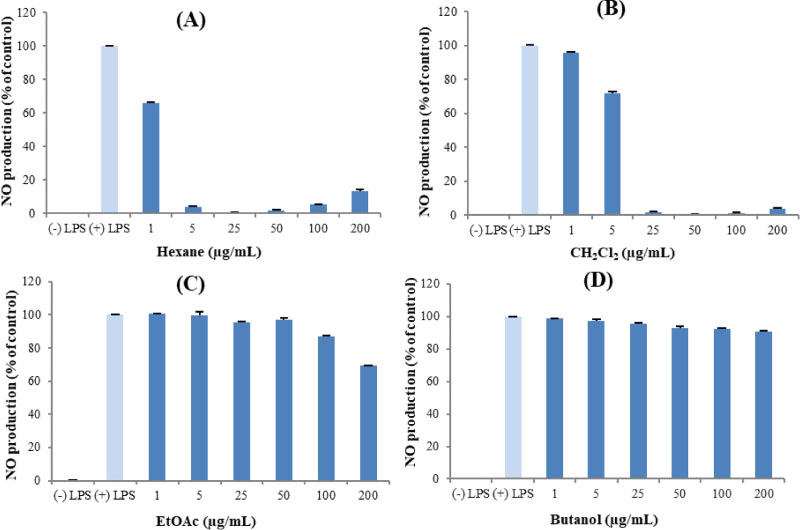
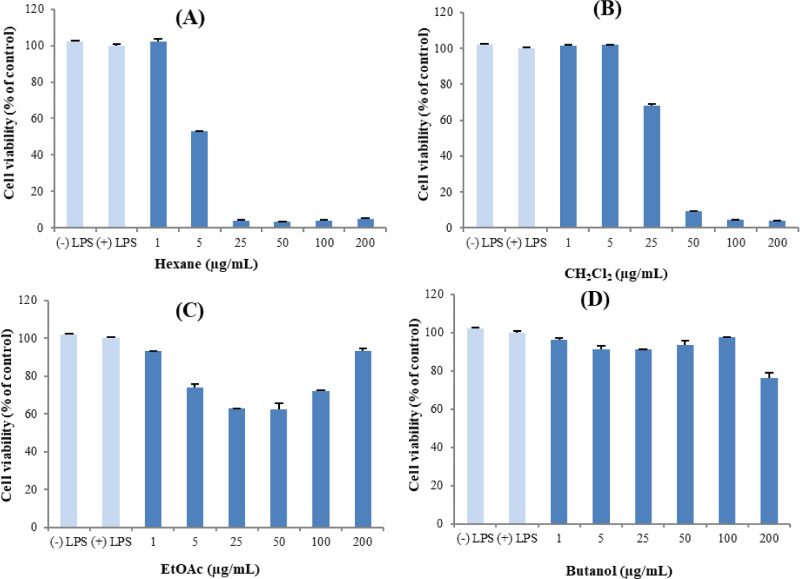
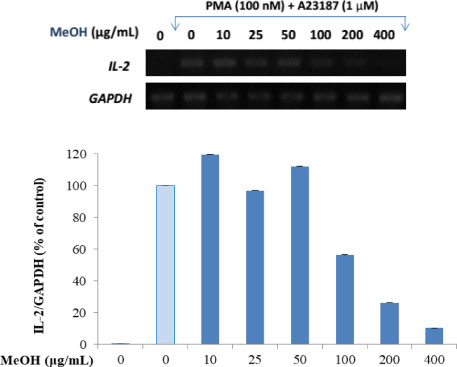
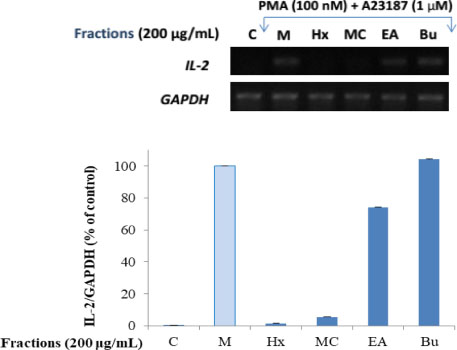
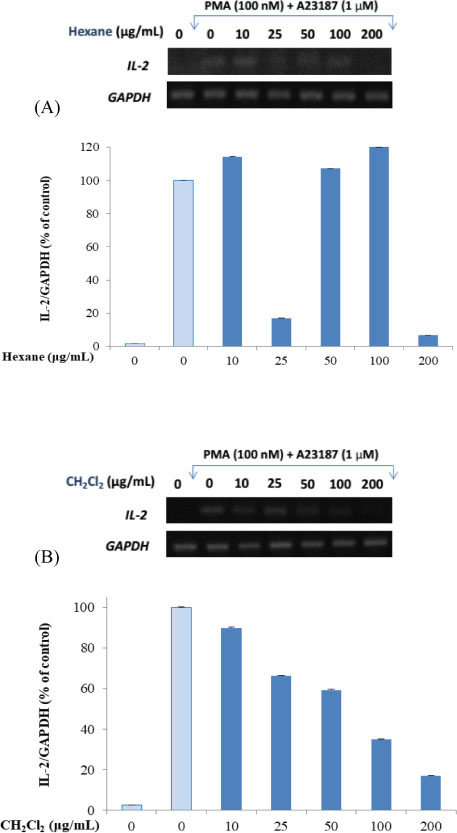
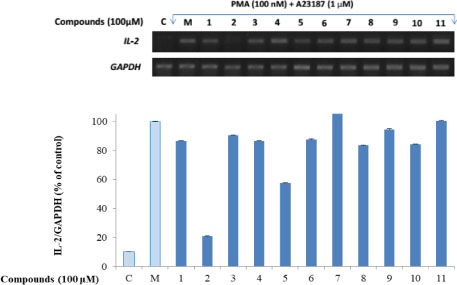
- Here, we designed to examine the anti-inflammatory effects on RAW264.7 cells and the immunosuppressive effects by evaluating interleukin-2 (IL-2) production in Jurkat T cells using a MeOH extract of Panax notoginseng roots. The results showed that the MeOH extract inhibited the synthesis of nitric oxide (NO) in a dose-dependent manner (ICâ‚…â‚€ value of 7.08 µg/mL) and displayed effects on T cell activation at a concentration of 400 µg/mL. In efforts to identify the potent compounds, bioactivity-guided fractionation of the MeOH extract and chemical investigation of its active CHâ‚‚Clâ‚‚-, EtOAc-, and butanol-soluble fractions led to the successful isolation and identification of eleven compounds, including two polyacetylenes (1, 2), a steroid saponin (3), seven dammarane-type ginsenosides (4 - 10), and an oleanane-type ginsenoside (11). Among them, compound 11 was isolated from this plant for the first time. Compound 2 exhibited potent inhibitory effects on NO synthesis and an immunosuppressive effect with ICâ‚…â‚€ values of 2.28 and 65.57 µM, respectively.
Keyword
MeSH Terms
Figure
Reference
-
1. Medzhitov R, Janeway CA Jr. Curr Opin Immunol. 1997; 9:4–9.2. Yang Z, Matteson EL, Goronzy JJ, Weyand CM. Arthritis Res Ther. 2015; 17:29.
Article3. Yu TK, Caudell EG, Smid C, Grimm EA. J Immunol. 2000; 164:6244–6251.4. Medzhitov R, Janeway CA Jr. Science. 2002; 296:298–300.5. Xu W, Larbi A. Exp Gerontol. 2018; 107:98–101.6. Wang CZ, McEntee E, Wicks S, Wu JA, Yuan CS. J Nat Med. 2006; 60:97–106.7. Lee DG, Lee J, Yang S, Kim KT, Lee S. Nat Prod Sci. 2015; 21:111–121.8. Lau AJ, Woo SO, Koh HL. J Chromatogr A. 2003; 1011:77–87.9. Ng TB. J Pharm Pharmacol. 2006; 58:1007–1019.10. Li SH, Chu Y. Acta Pharmacol Sin. 1999; 20:551–554.11. Zhao GR, Xiang ZJ, Ye TX, Yuan YJ, Guo ZX. Food Chem. 2006; 99:767–774.12. Ahn KS, Noh EJ, Zhao HL, Jung SH, Kang SS, Kim YS. Life Sci. 2005; 76:2315–2328.13. El-Desouky SK, Gamal-Eldeen AM. Pharm Biol. 2009; 47:872–877.14. Kim DC, Ko W, Ha TM, Nhiem NX, Yen PH, Tai BH, Truong LH, Long VN, Gioi T, Hong Quang T, Minh CV, Oh H, Kim YC, Kiem PV. Pharm Biol. 2017; 55:1195–1201.15. Min BS, Cuong TD. Nat Prod Sci. 2013; 19:201–205.16. Cao TQ, Tran MH, Kim JA, Tran PT, Lee JH, Woo MH, Lee HK, Min BS. Bioorg Med Chem Lett. 2015; 25:5087–5091.17. Knispel N, Ostrozhenkova E, Schramek N, Huber C, Peña-Rodríguez LM, Bonfill M, Palazón J, Wischmann G, Cusidó RM, Eisenreich W. Molecules. 2013; 18:7686–7698.18. Lee JM, Lee DG, Lee KH, Cho SH, Nam KW, Lee SH. Afr J Pharm Pharmacol. 2013; 7:294–301.19. Han M, Hou JG, Dong CM, Li W, Yu HL, Zheng YN, Chen L. Molecules. 2010; 15:399–406.20. Ko SR, Choi KJ, Suzuki K, Suzuki Y. Chem Pharm Bull. 2003; 51:404–408.21. Yu HS, Zhang LJ, Song XB, Liu YX, Zhang J, Cao M, Kang LP, Kang TG, Ma BP. China J Chin Mater Med. 2013; 38:3910–3917.22. Cho JG, Lee MK, Lee JW, Park HJ, Lee DY, Lee YH, Yang DC, Baek NI. J Ginseng Res. 2010; 34:113–121.23. Yang HJ, Kim JY, Kim SO, Yoo YH, Sung SH. J Ginseng Res. 2014; 38:194–202.24. Yoshikawa M, Matsuda H, Harada E, Murakami T, Wariishi N, Yamahara J, Murakami N. Chem Pharm Bull. 1994; 42:1354–1356.25. Ngo QT, Cao TQ, Tran PL, Kim JA, Seo ST, Kim JC, Woo MH, Lee JH, Min BS. Bioorg Med Chem Lett. 2018; 28:2109–2115.26. Robb RJ, Munck A, Smith KA. J Exp Med. 1981; 154:1455–1474.27. Hooton JW, Gibbs C, Paetkau V. J Immunol. 1985; 135:2464–2473.28. Muhlradt PF, Opitz HG. Eur J Immunol. 1982; 12:983–985.
Article29. Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA. J Immunol. 1985; 134:157–166.30. Shaw J, Meerovitch K, Bleackley RC, Paetkau V. J Immunol. 1988; 140:2243–2248.31. Pereira RL, Ibrahim T, Lucchetti L, da Silva AJ, Goncalves de Moraes VL. Immunopharmacology. 1999; 43:31–37.32. Jin L, Zhou W, Li R, Jin M, Jin C, Sun J, Li G. Nat Prod Res. 2019; 1–4.33. Christensen LP. Recent Pat Food Nutr Agric. 2011; 3:64–77.34. Chien SC, Tseng YH, Hsu WN, Chu FH, Chang ST, Kuo YH, Wang SY. Nat Prod Commun. 2014; 9:1589–1590.35. Shim SY, Sung SH, Lee M. Pharmacogn Mag. 2018; 14:358–362.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influence of Sam-Chil-Geun (Panax Notoginseng) on the Serum Lipid Levels and Inflammations of Rats with Hyperlipidemia Induced by Poloxamer-407
- The effect of Panax notoginseng saponins on oxidative stress induced by PCV2 infection in immune cells:in vitro and in vivo studies
- Changes of Prosapogenin Components in Tienchi Seng (Panax notoginseng) by Ultrasonic Thermal Fusion Process
- Inhibition of Arterial Myogenic Responses by a Mixed Aqueous Extract of Salvia Miltiorrhiza and Panax Notoginseng (PASEL) Showing Antihypertensive Effects
- Effects of AIF on Knee Osteoarthritis Patients: Double-blind, Randomized Placebo-controlled Study