Yonsei Med J.
2016 Jan;57(1):165-172. 10.3349/ymj.2016.57.1.165.
Basal Forebrain Cholinergic Deficits Reduce Glucose Metabolism and Function of Cholinergic and GABAergic Systems in the Cingulate Cortex
- Affiliations
-
- 1Brain Korea 21 PLUS Project for Medical Science & Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea. jchang@yuhs.ac changws0716@yuhs.ac
- 2Neuroscience Research Institute, Gachon University, Incheon, Korea.
- 3Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea. jchang@yuhs.ac changws0716@yuhs.ac
- KMID: 2466366
- DOI: http://doi.org/10.3349/ymj.2016.57.1.165
Abstract
- PURPOSE
Reduced brain glucose metabolism and basal forebrain cholinergic neuron degeneration are common features of Alzheimer's disease and have been correlated with memory function. Although regions representing glucose hypometabolism in patients with Alzheimer's disease are targets of cholinergic basal forebrain neurons, the interaction between cholinergic denervation and glucose hypometabolism is still unclear. The aim of the present study was to evaluate glucose metabolism changes caused by cholinergic deficits.
MATERIALS AND METHODS
We lesioned basal forebrain cholinergic neurons in rats using 192 immunoglobulin G-saporin. After 3 weeks, lesioned animals underwent water maze testing or were analyzed by 18F-2-fluoro-2-deoxyglucose positron emission tomography.
RESULTS
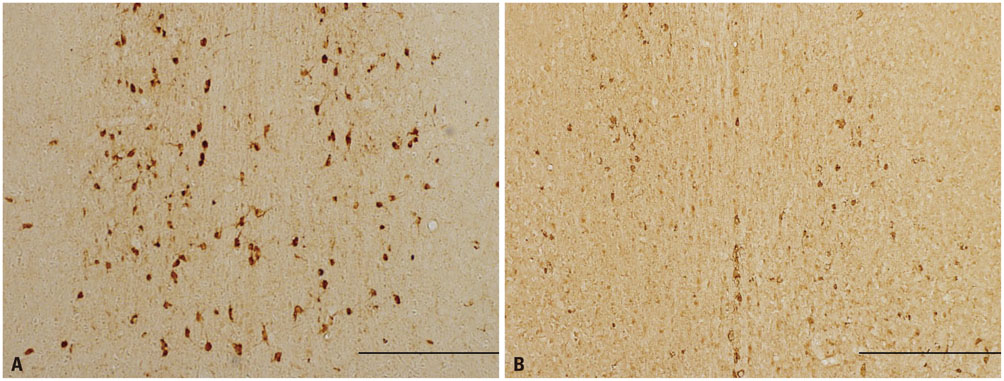
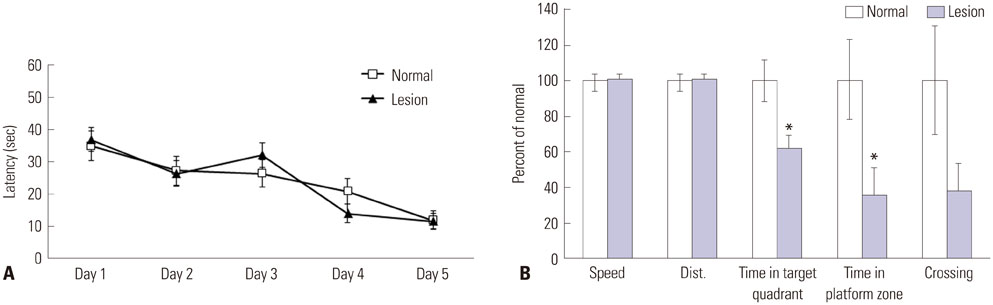
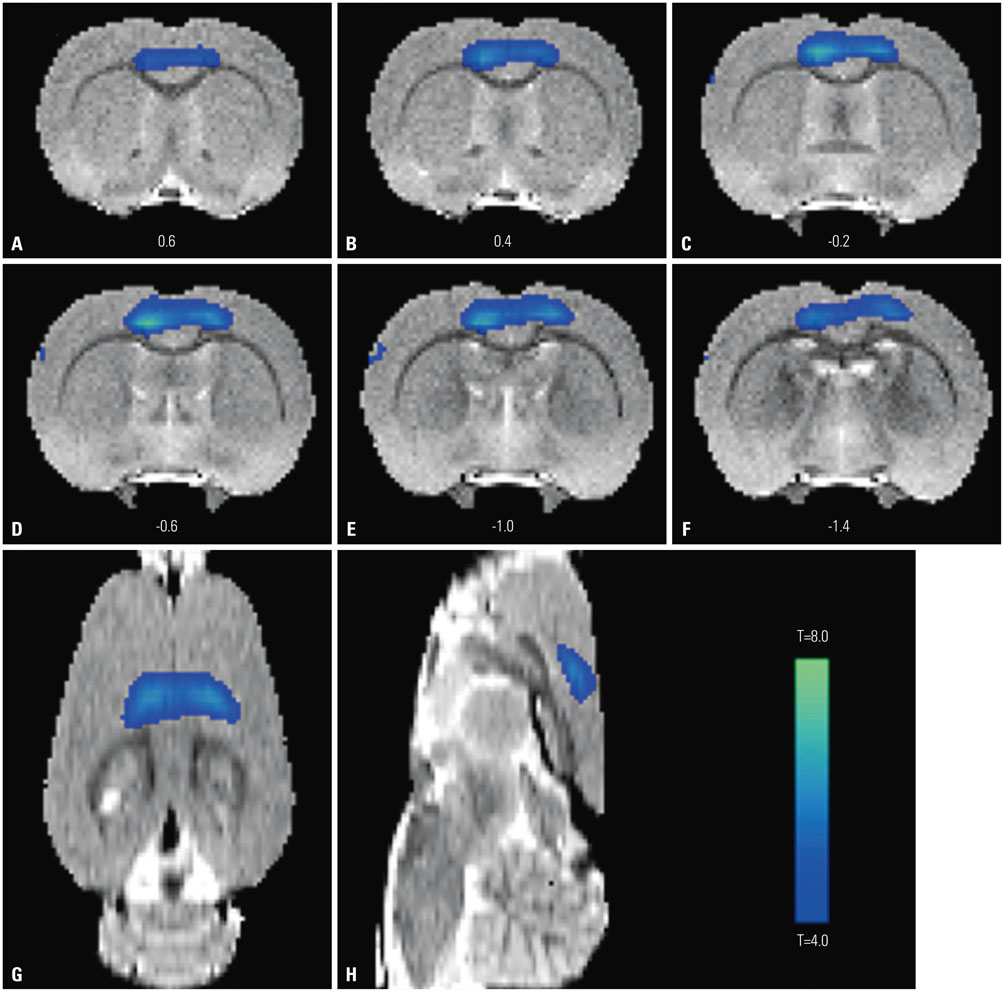
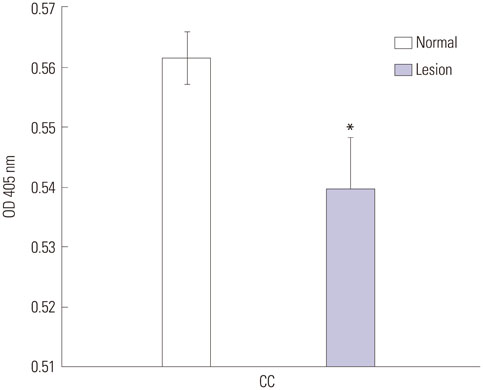
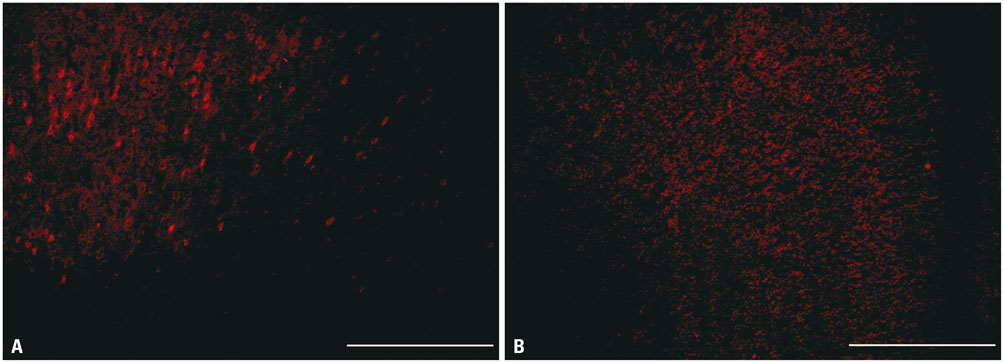
During water maze probe testing, performance of the lesioned group decreased with respect to time spent in the target quadrant and platform zone. Cingulate cortex glucose metabolism in the lesioned group decreased, compared with the normal group. Additionally, acetylcholinesterase activity and glutamate decarboxylase 65/67 expression declined in the cingulate cortex.
CONCLUSION
Our results reveal that spatial memory impairment in animals with selective basal forebrain cholinergic neuron damage is associated with a functional decline in the GABAergic and cholinergic system associated with cingulate cortex glucose hypometabolism.
MeSH Terms
-
Acetylcholine/metabolism
Alzheimer Disease
Animals
Antibodies, Monoclonal/*pharmacology
Basal Forebrain/*drug effects/metabolism
Cholinergic Agents/administration & dosage/*pharmacology
Cholinergic Neurons/*drug effects/metabolism
Fluorodeoxyglucose F18
GABAergic Neurons/*drug effects/metabolism
Glucose/*metabolism
Gyrus Cinguli/*drug effects/metabolism
Humans
Injections
Maze Learning
Motor Activity/physiology
Positron-Emission Tomography
Rats
Ribosome Inactivating Proteins, Type 1/*pharmacology
Acetylcholine
Antibodies, Monoclonal
Cholinergic Agents
Fluorodeoxyglucose F18
Glucose
Ribosome Inactivating Proteins, Type 1
Figure
Cited by 1 articles
-
Metabolism-Centric Overview of the Pathogenesis of Alzheimer's Disease
Somang Kang, Yong-ho Lee, Jong Eun Lee
Yonsei Med J. 2017;58(3):479-488. doi: 10.3349/ymj.2017.58.3.479.
Reference
-
1. Herholz K. PET studies in dementia. Ann Nucl Med. 2003; 17:79–89.
Article2. Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2009; 36:811–822.
Article3. Demetriades AK. Functional neuroimaging in Alzheimer's type dementia. J Neurol Sci. 2002; 203-204:247–251.
Article4. Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, et al. Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of the cholinergic deficits. J Neurochem. 1995; 64:749–760.
Article5. Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981; 10:122–126.
Article6. Nilsson OG, Leanza G, Rosenblad C, Lappi DA, Wiley RG, Björklund A. Spatial learning impairments in rats with selective immunolesion of the forebrain cholinergic system. Neuroreport. 1992; 3:1005–1008.
Article7. Croxson PL, Kyriazis DA, Baxter MG. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat Neurosci. 2011; 14:1510–1512.
Article8. Kiyosawa M, Baron JC, Hamel E, Pappata S, Duverger D, Riche D, et al. Time course of effects of unilateral lesions of the nucleus basalis of Meynert on glucose utilization by the cerebral cortex. Positron tomography in baboons. Brain. 1989; 112:435–455.
Article9. Harrell LE, Davis JN. Cholinergic denervation of the hippocampal formation does not produce long-term changes in glucose metabolism. Exp Neurol. 1984; 85:128–138.
Article10. Ouchi Y, Fukuyama H, Ogawa M, Yamauchi H, Kimura J, Magata Y, et al. Cholinergic projection from the basal forebrain and cerebral glucose metabolism in rats: a dynamic PET study. J Cereb Blood Flow Metab. 1996; 16:34–41.
Article11. Browne SE, Lin L, Mattsson A, Georgievska B, Isacson O. Selective antibody-induced cholinergic cell and synapse loss produce sustained hippocampal and cortical hypometabolism with correlated cognitive deficits. Exp Neurol. 2001; 170:36–47.
Article12. Jeong da U, Chang WS, Hwang YS, Lee D, Chang JW. Decrease of GABAergic markers and arc protein expression in the frontal cortex by intraventricular 192 IgG-saporin. Dement Geriatr Cogn Disord. 2011; 32:70–78.
Article13. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego (CA): Academic Press;1998.14. Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961; 7:88–95.
Article15. Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods. 2003; 129:105–113.
Article16. Stewart DJ, MacFabe DF, Leung LW. Topographical projection of cholinergic neurons in the basal forebrain to the cingulate cortex in the rat. Brain Res. 1985; 358:404–407.
Article17. Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982; 8:727–749.
Article18. Katsumi Y, Hanakawa T, Fukuyama H, Hayashi T, Nagahama Y, Yamauchi H, et al. The effect of sequential lesioning in the basal forebrain on cerebral cortical glucose metabolism in rats. An animal positron emission tomography study. Brain Res. 1999; 837:75–82.
Article19. Ogawa M, Iida Y, Nakagawa M, Kuge Y, Kawashima H, Tominaga A, et al. Change of central cholinergic receptors following lesions of nucleus basalis magnocellularis in rats: search for an imaging index suitable for the early detection of Alzheimer's disease. Nucl Med Biol. 2006; 33:249–254.
Article20. Katsumi Y, Hayashi T, Oyanagi C, Nagahama Y, Yamauchi H, Ono S, et al. Glucose metabolism in the rat frontal cortex recovered without the recovery of choline acetyltransferase activity after lesioning of the nucleus basalis magnocellularis. Neurosci Lett. 2000; 280:9–12.
Article21. Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002; 35:625–641.
Article22. Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004; 5:361–372.
Article23. Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999; 400:675–677.
Article24. Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001; 411:309–313.
Article25. Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006; 26:7555–7564.
Article26. Liu F, Zheng XL, Li BM. The anterior cingulate cortex is involved in retrieval of long-term/long-lasting but not short-term memory for step-through inhibitory avoidance in rats. Neurosci Lett. 2009; 460:175–179.
Article27. Weible AP, Rowland DC, Monaghan CK, Wolfgang NT, Kentros CG. Neural correlates of long-term object memory in the mouse anterior cingulate cortex. J Neurosci. 2012; 32:5598–5608.
Article28. Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010; 68:521–534.
Article29. Bijak M. Daily changes in the mouse cingulate cortex responsivity to neurotransmitters and depolarizing medium: an extracellular in vitro study. Gen Pharmacol. 1988; 19:107–111.
Article30. Jones RS, Olpe HR. Multiple changes in the sensitivity of cingulate cortical neurones to putative neurotransmitters in ageing rats: substance P, acetylcholine and noradrenaline. Neurosci Lett. 1984; 50:31–36.
Article31. Farr SA, Uezu K, Creonte TA, Flood JF, Morley JE. Modulation of memory processing in the cingulate cortex of mice. Pharmacol Biochem Behav. 2000; 65:363–368.
Article32. Kohl MM, Paulsen O. The roles of GABAB receptors in cortical network activity. Adv Pharmacol. 2010; 58:205–229.
Article33. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994; 91:10625–10629.
Article34. Reine G, Samuel D, Nieoullon A, Kerkerian-Le Goff L. Effects of lesion of the cholinergic basal forebrain nuclei on the activity of glutamatergic and GABAergic systems in the rat frontal cortex and hippocampus. J Neural Transm Gen Sect. 1992; 87:175–192.
Article35. Rossner S, Schliebs R, Bigl V. 192IgG-saporin-induced immunotoxic lesions of cholinergic basal forebrain system differentially affect glutamatergic and GABAergic markers in cortical rat brain regions. Brain Res. 1995; 696:165–176.
Article36. Nicolle MM, Shivers A, Gill TM, Gallagher M. Hippocampal N-methyl-D-aspartate and kainate binding in response to entorhinal cortex aspiration or 192 IgG-saporin lesions of the basal forebrain. Neuroscience. 1997; 77:649–659.
Article37. Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002; 12:386–397.
Article38. Alexopoulos GS, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Murphy CF. Anterior cingulate dysfunction in geriatric depression. Int J Geriatr Psychiatry. 2008; 23:347–355.
Article39. Toyota Y, Ikeda M, Shinagawa S, Matsumoto T, Matsumoto N, Hokoishi K, et al. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer's disease. Int J Geriatr Psychiatry. 2007; 22:896–901.
Article40. Iqbal K, Liu F, Gong CX. Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem Pharmacol. 2014; 88:631–639.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- beta-Amyloid Neurotoxicity on Basal Forebrain Cholinergic Neuron Cultures
- Basal Forebrain Cholinergic-induced Activation of Cholecystokinin Inhibitory Neurons in the Basolateral Amygdala
- Effects on 5-HT and Ach in the Rat by the I.C.V Injection of PCPA
- The effect of electroconvulsive shock and cholinergic drugs on anterograde amnesia in albino rats
- Muscarine M2 Receptor-mediated Presynaptic Inhibition of GABAergic Transmission in Rat Meynert Neurons