J Korean Ophthalmol Soc.
2019 Dec;60(12):1162-1168. 10.3341/jkos.2019.60.12.1162.
Clinical Manifestations and Outcomes of Varicella-zoster Virus Endotheliitis
- Affiliations
-
- 1Department of Ophthalmology, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea. maya12kim@naver.com
- 2Health Science Institute, Gyeongsang National University, Jinju, Korea.
- KMID: 2466166
- DOI: http://doi.org/10.3341/jkos.2019.60.12.1162
Abstract
- PURPOSE
We evaluated the clinical manifestations of varicella-zoster virus (VZV)-induced endotheliitis and treatment outcomes.
METHODS
We retrospectively reviewed the medical records of patients exhibiting clinical manifestations of endotheliitis diagnosed as VZV endotheliitis via polymerase chain reaction (PCR) of anterior chamber puncture fluid from January 2013 to December 2018. Their clinical characteristics, treatments, and outcomes were analyzed.
RESULTS
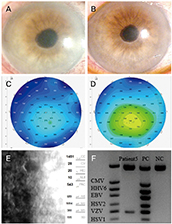
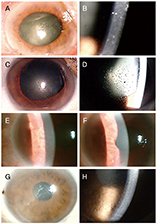
Seven eyes of seven patients were diagnosed as VZV-affected via PCR of the aqueous humor. Mean patient age was 70.4 ± 10.4 years and the average follow-up time 24.7 ± 3.8 months. All eyes exhibited mild anterior chamber inflammation (trace to 1+). Four eyes were disciform in shape and three exhibited diffuse endotheliitis. Six patients evidenced intraocular pressures >21 mmHg. All patients were treated with oral antiviral agents; they were cured and no recurrence was noted. The mean best-corrected visual acuity (logMAR) increased significantly from 0.73 ± 0.19 to 0.09 ± 0.07 and the mean ocular pressure decreased significantly from 26.1 ± 7.3 to 13.2 ± 2.1 mmHg.
CONCLUSIONS
VZV endotheliitis may present as mild inflammation of the anterior chamber with a disciform eye or diffuse corneal edema. Diagnosis is aided by VZV-specific PCR of anterior chamber fluid; oral antiviral agents are useful. Be diagnosed with PCR of anterior chamber, and be treated with oral antiviral agents.
Keyword
MeSH Terms
Figure
Reference
-
1. Suzuki T, Ohashi Y. Corneal endotheliitis. Semin Ophthalmol. 2008; 23:235–240.2. Koizumi N, Yamasaki K, Kawasaki S, et al. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am J Ophthalmol. 2006; 141:564–565.3. Koizumi N, Inatomi T, Suzuki T, et al. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan corneal endotheliitis study. Br J Ophthalmol. 2015; 99:54–58.4. Khodabande A. Varicella endotheliitis: a case report. Eur J Ophthalmol. 2009; 19:1076–1078.5. de Freitas D, Sato EH, Kelly LD, Pavan-Langston D. Delayed onset of varicella keratitis. Cornea. 1992; 11:471–474.6. Sutcliffe E, Baum J. Acute idiopathic corneal endotheliitis. Trans Am Ophthalmol Soc. 1983; 81:86–96.7. Peng RM, Guo YX, Xiao GG, et al. Clinical manifestations and characteristics of in vivo confocal microscopy in varicella zoster virus-related corneal endotheliitis. Ocul immunol inflamm. 2019; 27:1270–1279.8. Kim YJ, Yoo WS, Han YS, et al. Clinical manifestations and outcomes of treatment in cytomegalovirus endotheliitis. J Korean Ophthalmol Soc. 2016; 57:863–875.9. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008; 115(2 Suppl):S3–S12.10. Bandeira F, Roizenblatt M, Levi GC, et al. Herpes zoster ophthalmicus and varicella zoster virus vasculopathy. Arq Bras Oftalmol. 2016; 79:126–129.11. Mun CY, Jung MS. Clinical features and risk factors of herpes zoster ophthalmicus. J Korean Ophthalmol Soc. 2017; 58:1317–1324.12. Takase H, Kubono R, Terada Y, et al. Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol. 2014; 58:473–482.13. Sakai JI, Usui Y, Suzuki J, et al. Clinical features of anterior uveitis caused by three different herpes viruses. Int Ophthalmol. 2019; 05. 27. DOI: 10.1007/s10792-019-01125-5. [Epub ahead of print].14. Tugal-Tutkun I, Cimino L, Akova YA. Review for disease of the year: varicella zoster virus-induced anterior uveitis. Ocul Immunol inflamm. 2018; 26:171–177.15. Huff JC, Bean B, Balfour HH Jr, et al. Therapy of herpes zoster with oral acyclovir. Am J Med. 1988; 85:84–89.16. McKendrick MW, McCil JI, White JE, Wood MJ. Oral acyclovir in acute herpes zoster. Br Med J (Clin Res Ed). 1986; 293:1529–1532.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diverse clinical manifestations caused by varicella-zoster virus reactivation
- Clinical Significance of Serum Varicella Zoster Virus Immunoglobulin M and G in Varicella and Herpes Zoster
- Herpes zoster complicated by deep vein thrombosis : a case report
- Association of Herpes Zoster and Lymphosarcoma: Report of one Case
- A clinical study on varicella zoster virus infection and treatment in children with malignant lymphoproliferative disease