Int J Stem Cells.
2019 Jul;12(2):195-205. 10.15283/ijsc18076.
Efficacies of Stem Cell Therapies for Functional Improvement of the β Cell in Patients with Diabetes: A Systematic Review of Controlled Clinical Trials
- Affiliations
-
- 1The Catholic University of Korea, Catholic Medical Center, Seoul, Korea.
- 2Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. y1693@catholic.ac.kr
- 3Cell and Gene Therapy Products Division, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Cheongju, Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Medical Library, The Catholic University of Korea, Seoul, Korea.
- 6Catholic High-Performance Cell Therapy Center & Department of Medical Life Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2465891
- DOI: http://doi.org/10.15283/ijsc18076
Abstract
- BACKGROUND AND OBJECTIVES
This study was performed to investigate whether stem cell therapy enhances β cell function by meta-analysis with proper consideration of variability of outcome measurements in controlled trial of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) patients.
METHODS
A systematic search was performed from inception to January 2018 in PubMed, EMBASE, and Cochrane databases. β cell function was assessed by stimulated C-peptide, fasting C-peptide, normal glycosylated hemoglobin levels (HbA1C), and exogenous insulin dose patterns. The quality of the studies were assessed by both the Cochrane Collaboration's Risk of Bias (ROB) for Randomized controlled trials and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) for non-randomized controlled trials.
RESULTS
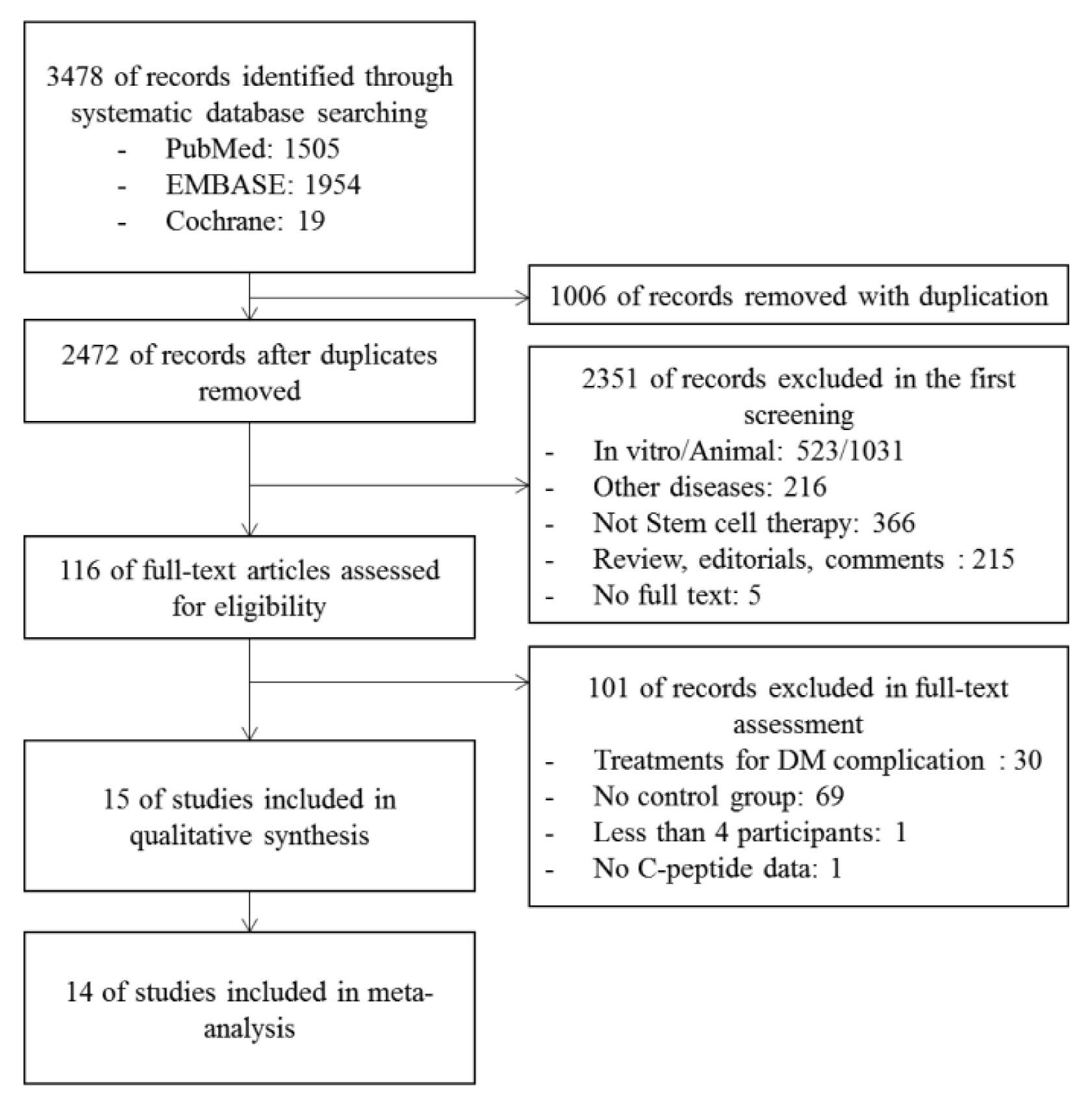
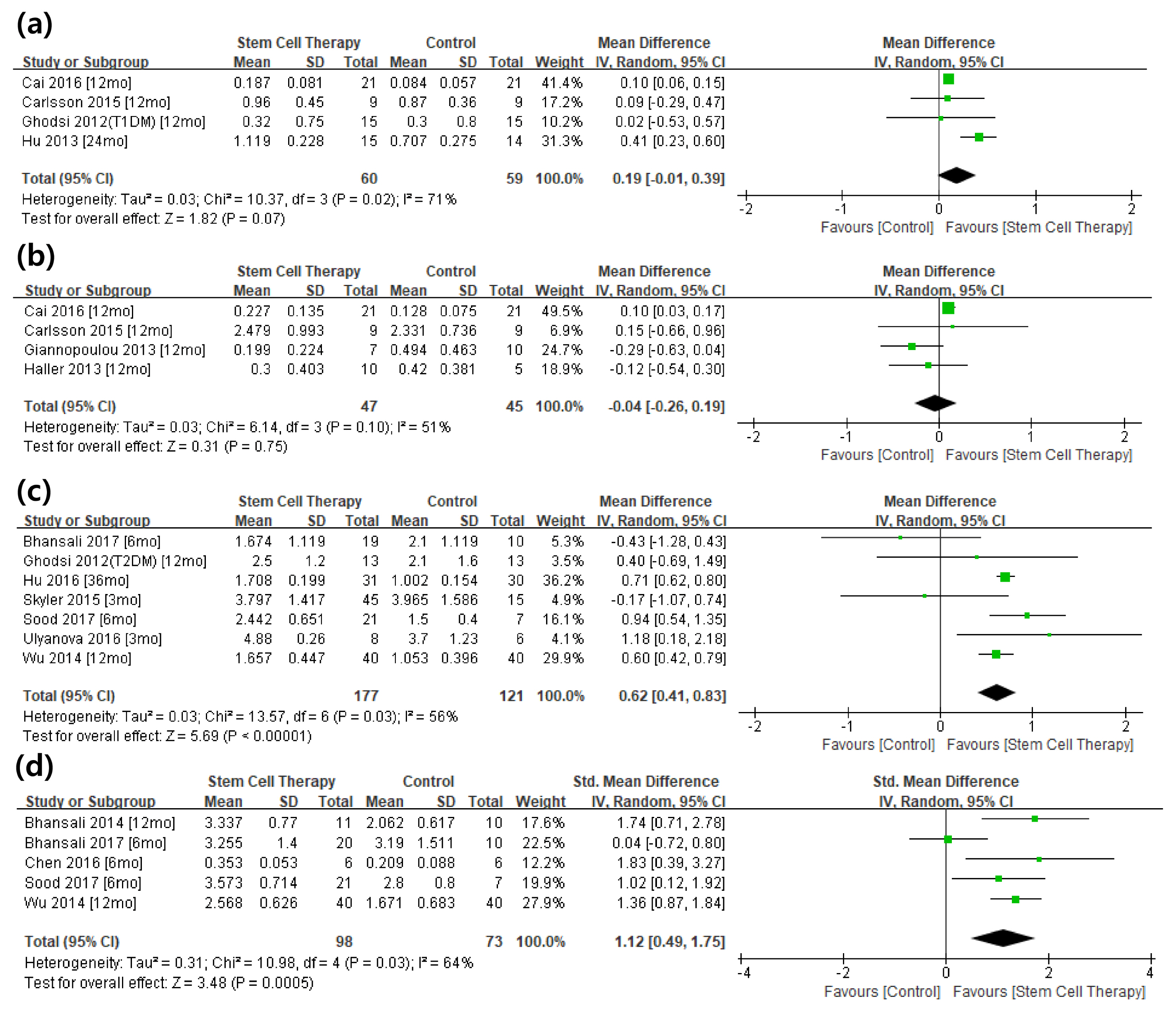
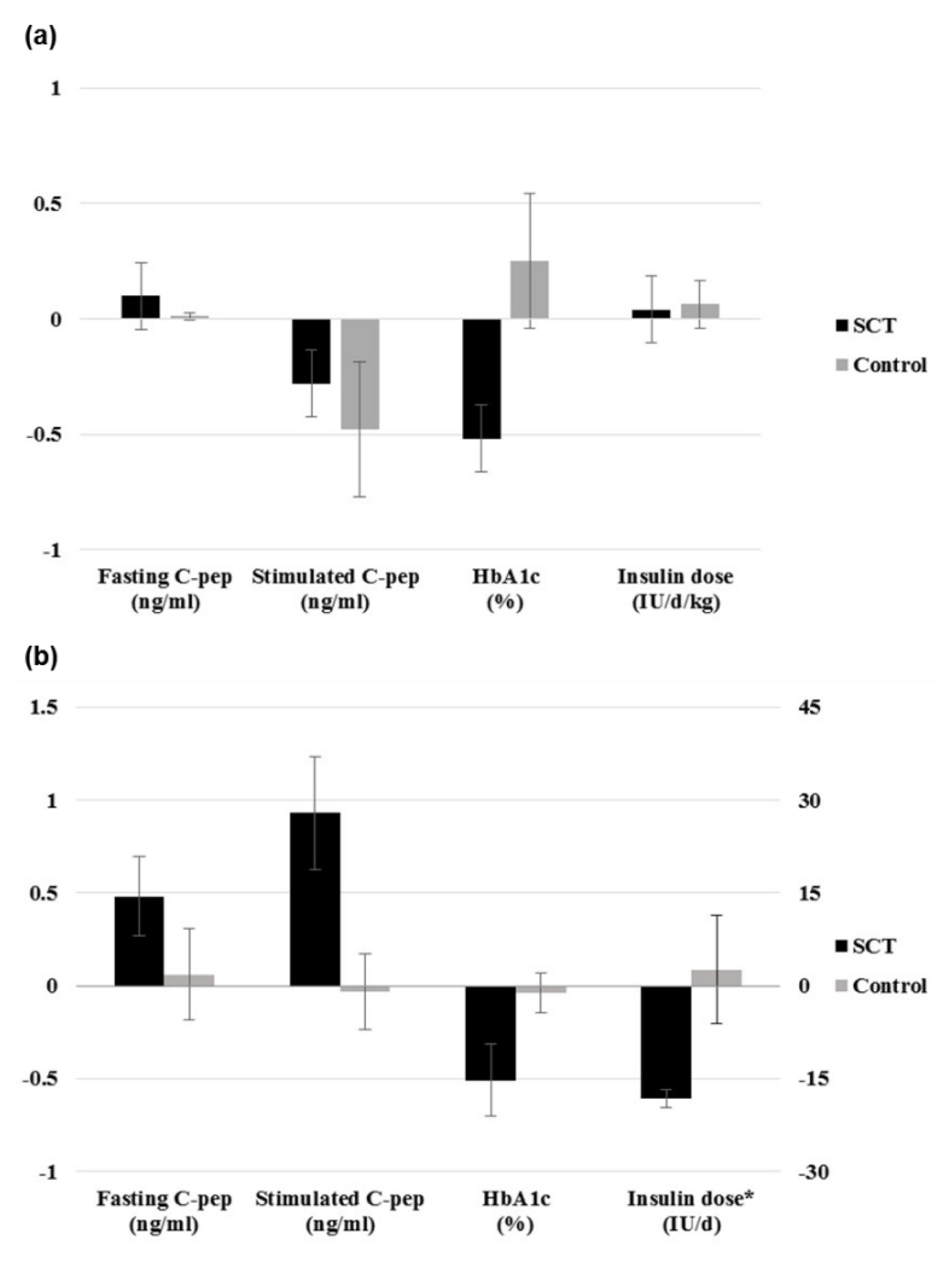
From the selected final 15 articles, total of 16 trials were analyzed. There were 6 T1DM trials (total 153 cases) and 10 T2DM trials (total 457 cases). In T2DM patients, the changes in stimulated C-peptide, HbA1c, and exogenous insulin dose versus baseline showed a favorable pattern with a significant heterogeneity in stem cell therapy. In T1DM, there was no significant difference between control group and stem cell therapy group in three indicators except for HbA1c. Most of the studies were rated as having high risk of bias in the quality assessment.
CONCLUSIONS
The stem cell therapy for DM patients is not effective in T1DM but seems to be effective in improving the β cell function in T2DM. However the observed effect should be interpreted with caution due to the significant heterogeneity and high risk of bias within the studies. Further verification through a rigorously designed study is warranted.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998; 128:517–523. DOI: 10.7326/0003-4819-128-7-199804010-00001. PMID: 9518395.2. Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001; 86:4047–4058. PMID: 11549624.
Article3. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995; 44:1249–1258. DOI: 10.2337/diab.44.11.1249.4. Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med. 1998; 15:290–296. DOI: 10.1002/(SICI)1096-9136(199804)15:4<290::AID-DIA570>3.0.CO;2-M. PMID: 9585393.
Article5. Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003; 96:281–288. DOI: 10.1093/qjmed/hcg040. PMID: 12651972.
Article6. Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007; 297:1568–1576. DOI: 10.1001/jama.297.14.1568. PMID: 17426276.
Article7. Couri CE, de Oliveira MC, Simões BP. Risks, benefits, and therapeutic potential of hematopoietic stem cell transplantation for autoimmune diabetes. Curr Diab Rep. 2012; 12:604–611. DOI: 10.1007/s11892-012-0309-0. PMID: 22864730.
Article8. Snarski E, Milczarczyk A, Torosian T, Paluszewska M, Urbanowska E, Król M, Boguradzki P, Jedynasty K, Franek E, Wiktor-Jedrzejczak W. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant. 2011; 46:562–566. DOI: 10.1038/bmt.2010.147. PMID: 20581881.
Article9. Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, Echevarria L, Froud T, Bernetti K, Cayetano SM, Velazquez O, Alejandro R, Ricordi C. Combined treatment of intra-pancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008; 17:1295–1304. DOI: 10.3727/096368908787648119.
Article10. Bhansali S, Dutta P, Kumar V, Yadav MK, Jain A, Mudaliar S, Bhansali S, Sharma RR, Jha V, Marwaha N, Khandelwal N, Srinivasan A, Sachdeva N, Hawkins M, Bhansali A. Efficacy of autologous bone marrow-derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: a randomized, placebo-controlled comparative study. Stem Cells Dev. 2017; 26:471–481. DOI: 10.1089/scd.2016.0275. PMID: 28006991.
Article11. Ghodsi M, Heshmat R, Amoli M, Keshtkar AA, Arjmand B, Aghayan H, Hosseini P, Sharifi AM, Larijani B. The effect of fetal liver-derived cell suspension allotransplantation on patients with diabetes: first year of follow-up. Acta Med Iran. 2012; 50:541–546. PMID: 23109026.12. Cernea S, Raz I, Herold KC, Hirshberg B, Roep BO, Schatz DA, Fleming GA, Pozzilli P, Little R, Schloot NC, Leslie RD, Skyler JS, Palmer JP. D-Cure Workshop. Challenges in developing endpoints for type 1 diabetes intervention studies. Diabetes Metab Res Rev. 2009; 25:694–704. DOI: 10.1002/dmrr.1002. PMID: 19771545.
Article13. Greenbaum CJ, Harrison LC. Immunology of Diabetes Society. Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes. 2003; 52:1059–1065. DOI: 10.2337/diabetes.52.5.1059. PMID: 12716733.
Article14. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004; 53:250–264. DOI: 10.2337/diabetes.53.1.250. PMID: 14693724.
Article15. Wang ZX, Cao JX, Li D, Zhang XY, Liu JL, Li JL, Wang M, Liu Y, Xu BL, Wang HB. Clinical efficacy of autologous stem cell transplantation for the treatment of patients with type 2 diabetes mellitus: a meta-analysis. Cytotherapy. 2015; 17:956–968. DOI: 10.1016/j.jcyt.2015.02.014. PMID: 25824289.
Article16. El-Badawy A, El-Badri N. clinical efficacy of stem cell therapy for diabetes mellitus: a meta-analysis. PLoS One. 2016; 11:e0151938. DOI: 10.1371/journal.pone.0151938. PMID: 27073927. PMCID: 4830527.
Article17. Cao JX, Zhao YQ, Ding GC, Li JL, Liu YS, Wang M, Xu BL, Liu JL, Wang ZX. Evaluation of the clinical efficacy of stem cell transplantation in patients with type 1 diabetes mellitus. Int J Clin Exp Med. 2016; 9:19034–19051.18. Ulyanova O, Taubaldieva Z, Tuganbekova S, Saparbayev S, Kim N, Trimova R, Kozina L, Shaimardanova G. Leptin level in patients with type 2 diabetes mellitus after fetal pancreatic stem cell transplant. Exp Clin Transplant. 2016; 14(Suppl 3):45–47. PMID: 27805510.19. Chen P, Huang Q, Xu XJ, Shao ZL, Huang LH, Yang XZ, Guo W, Li CM, Chen C. The effect of liraglutide in combination with human umbilical cord mesenchymal stem cells treatment on glucose metabolism and β cell function in type 2 diabetes mellitus. Chin J Int Med. 2016; 55:349–354. Chinese. DOI: 10.3760/cma.j.issn.0578-1426.2016.05.004. PMID: 27143183.20. Wu Z, Cai J, Chen J, Huang L, Wu W, Luo F, Wu C, Liao L, Tan J. Autologous bone marrow mononuclear cell infusion and hyperbaric oxygen therapy in type 2 diabetes mellitus: an open-label, randomized controlled clinical trial. Cytotherapy. 2014; 16:258–265. DOI: 10.1016/j.jcyt.2013.10.004. PMID: 24290656.
Article21. Sood V, Bhansali A, Mittal BR, Singh B, Marwaha N, Jain A, Khandelwal N. Autologous bone marrow derived stem cell therapy in patients with type 2 diabetes mellitus - defining adequate administration methods. World J Diabetes. 2017; 8:381–389. DOI: 10.4239/wjd.v8.i7.381. PMID: 28751962. PMCID: 5507836.
Article22. Skyler JS, Fonseca VA, Segal KR, Rosenstock J. MSB-DM003 Investigators. Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care. 2015; 38:1742–1749. DOI: 10.2337/dc14-2830. PMID: 26153271. PMCID: 4542273.
Article23. Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, Chen Y, Zhao W, Jia Z, Yan S, Wang Y. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013; 60:347–357. DOI: 10.1507/endocrj.EJ12-0343. PMID: 23154532.
Article24. Hu J, Wang Y, Gong H, Yu C, Guo C, Wang F, Yan S, Xu H. Long term effect and safety of Wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes. Exp Ther Med. 2016; 12:1857–1866. DOI: 10.3892/etm.2016.3544. PMID: 27588104. PMCID: 4997981.
Article25. Haller MJ, Wasserfall CH, Hulme MA, Cintron M, Brusko TM, McGrail KM, Wingard JR, Theriaque DW, Shuster JJ, Ferguson RJ, Kozuch M, Clare-Salzler M, Atkinson MA, Schatz DA. Autologous umbilical cord blood infusion followed by oral docosahexaenoic acid and vitamin D supplementation for C-peptide preservation in children with Type 1 diabetes. Biol Blood Marrow Transplant. 2013; 19:1126–1129. DOI: 10.1016/j.bbmt.2013.04.011. PMID: 23611977. PMCID: 3852705.
Article26. Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015; 64:587–592. DOI: 10.2337/db14-0656. PMID: 25204974.
Article27. Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, Wu W, Luo F, Wu C, Pugliese A, Pileggi A, Ricordi C, Tan J. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. diabetes care. 2016; 39:149–157. DOI: 10.2337/dc15-0171. PMID: 26628416.
Article28. Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, Sachdeva N, Sharma RR, Marwaha N, Khandelwal N. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014; 23:1075–1085. DOI: 10.3727/096368913X665576. PMID: 23561959.
Article29. Giannopoulou EZ, Puff R, Beyerlein A, von Luettichau I, Boerschmann H, Schatz D, Atkinson M, Haller MJ, Egger D, Burdach S, Ziegler AG. Effect of a single autologous cord blood infusion on beta-cell and immune function in children with new onset type 1 diabetes: a non-randomized, controlled trial. Pediatr Diabetes. 2014; 15:100–109. DOI: 10.1111/pedi.12072. PMID: 24102806.
Article30. Hu J, Li C, Wang L, Zhang X, Zhang M, Gao H, Yu X, Wang F, Zhao W, Yan S, Wang Y. Long term effects of the implantation of autologous bone marrow mononuclear cells for type 2 diabetes mellitus. Endocr J. 2012; 59:1031–1039. DOI: 10.1507/endocrj.EJ12-0092. PMID: 22814142.
Article31. Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of β-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care. 2013; 36:2324–2330. DOI: 10.2337/dc12-0607. PMID: 23564921. PMCID: 3714535.
Article32. King AB. Misled by the morning “fasting” plasma glucose. J Diabetes Sci Technol. 2015; 9:1342–1345. DOI: 10.1177/1932296815586425. PMID: 25972281. PMCID: 4667307.
Article33. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013; 30:803–817. DOI: 10.1111/dme.12159. PMID: 23413806. PMCID: 3748788.
Article34. Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004; 364:203–205. DOI: 10.1016/S0140-6736(04)16635-X. PMID: 15246735.
Article35. Huss R, Xiangwei X, Heimberg H. Adult stem cells regenerate the endocrine pankreas and normalize hyperglycaemia and insulin production in diabetic mice. Verh Dtsch Ges Pathol. 2005; 89:184–190. German. PMID: 18035689.36. Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, Li H, Zhang Y, Diao Y, Li Y, Chen Y, Sun X, Fisk MB, Skidgel R, Holterman M, Prabhakar B, Mazzone T. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012; 10:3. DOI: 10.1186/1741-7015-10-3. PMID: 22233865. PMCID: PMC3322343.
Article37. Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Zhou H, Yin Z, Chen Y, Zhang Y, Wang S, Shen J, Thaker H, Jain S, Li Y, Diao Y, Chen Y, Sun X, Fisk MB, Li H. Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells (CB-SCs) in stem cell educator therapy: phase I/II clinical trial. BMC Med. 2013; 11:160. DOI: 10.1186/1741-7015-11-160. PMID: 23837842. PMCID: 3716981.
Article38. Cantú-Rodríguez OG, Lavalle-Gonzalez F, Herrera-Rojas MA, Gutiérrez-Aguirre CH, Mancías-Guerra C, Jaime-Pérez JC, Gonzalez-Llano O, Zapata-Garrido A, Villarreal-Perez JZ, Gomez-Almaguer D. Autologus hematopoietic stem cell transplant in type 1 diabetes mellitus in a nonmyeloablative and outpatient setting. Blood. 2014; 124:1191.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke
- Adult Stem Cell Therapy for Stroke: Challenges and Progress
- Ethical Issues in Stem Cell Therapy
- Current Concepts in Stem Cell Therapy for Cardiovascular Diseases: What We Know and Don't Know
- Advances in laser and stem cell treatment: current technologies, limitations, and future prospects