J Pathol Transl Med.
2019 Mar;53(2):86-93. 10.4132/jptm.2018.12.26.
Human Leukocyte Antigen Class I and Programmed Death-Ligand 1 Coexpression Is an Independent Poor Prognostic Factor in Adenocarcinoma of the Lung
- Affiliations
-
- 1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea. chungjh@snu.ac.kr
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2465447
- DOI: http://doi.org/10.4132/jptm.2018.12.26
Abstract
- BACKGROUND
Both human leukocyte antigen (HLA) class I and programmed death-ligand 1 (PD-L1) molecules are known to play important roles in cancer immunity. In this study, we evaluated HLA class I expression in resected adenocarcinoma of the lung, and investigated its prognostic impact in correlation with PD-L1 expression.
METHODS
HLA class I and PD-L1 expression was evaluated by immunohistochemistry in a total of 403 resected lung adenocarcinomas using tissue microarray. Correlations between the expression of HLA class I/PD-L1 and clinicopathologic features and prognostic significance were analyzed.
RESULTS
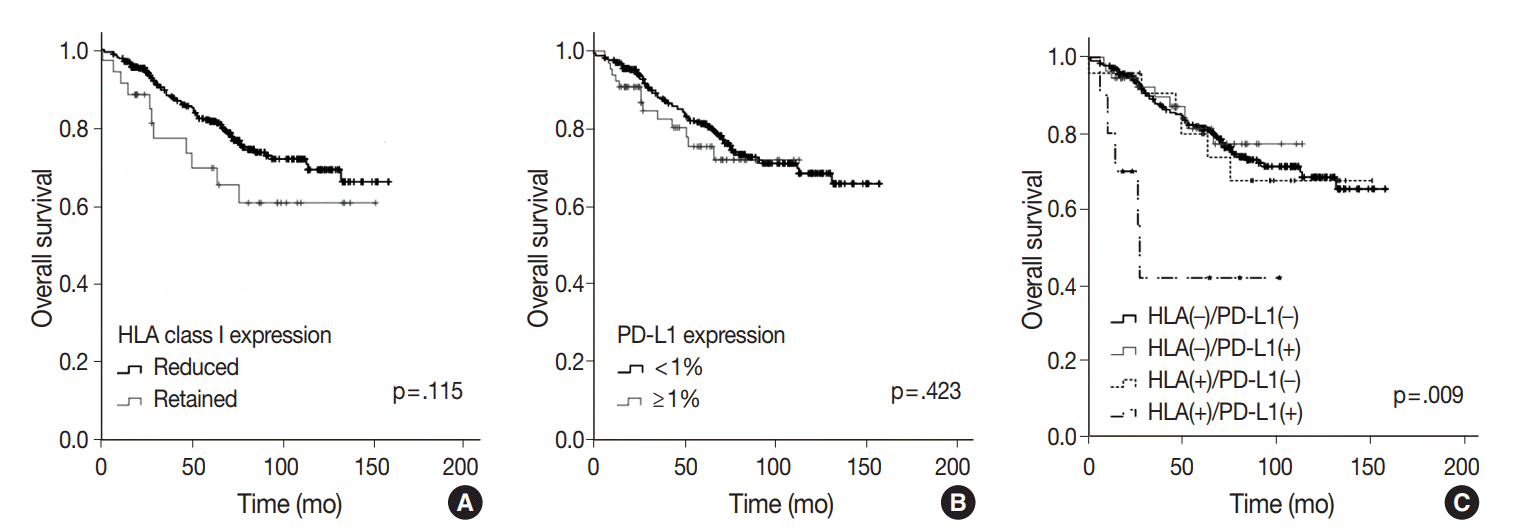
HLA class I expression was reduced in 91.6% of adenocarcinoma, and more frequently reduced in patients with younger age, absence of vascular invasion, and low pathologic stage (p = .033, p = .007, and p = .012, respectively). Positive PD-L1 expression in tumor cells was 16.1% (1% cut-off), and associated with poor differentiation, presence of vascular invasion and nodal metastasis (p < .001, p = .002, and p = .032, respectively). On survival analysis, HLA class I or PD-L1 expression alone did not show any statistical significance. On the integrated analysis, HLA class I (+)/PD-L1 (+) subgroup showed a significantly shorter overall survival than other groups (p = .001). Multivariate analysis revealed that coexpression of HLA class I and PD-L1 was an independent poor prognostic factor of lung adenocarcinoma. (p < .001; hazard ratio, 6.106; 95% confidence interval, 2.260 to 16.501).
CONCLUSIONS
Lung adenocarcinoma with coexpression of HLA class I and PD-L1 was associated with poor prognosis. This subgroup may evade immune attack by expressing PD-L1 protein despite HLA expression.
Keyword
MeSH Terms
Figure
Reference
-
1. Shim HS, Choi YL, Kim L, et al. Molecular testing of lung cancers. J Pathol Transl Med. 2017; 51:242–54.
Article2. Torre LA, Sauer AM, Chen MS Jr, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: converging incidence in males and females. CA Cancer J Clin. 2016; 66:182–202.
Article3. Somasundaram A, Burns TF. The next generation of immunotherapy: keeping lung cancer in check. J Hematol Oncol. 2017; 10:87.
Article4. Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res. 2015; 4:177–90.5. Concha-Benavente F, Srivastava R, Ferrone S, Ferris RL. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016; 58:52–8.
Article6. Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008; 27:5869–85.
Article7. Hirai A, Yoneda K, Shimajiri S, et al. Prognostic impact of programmed death-ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J Thorac Cardiovasc Surg. 2018; 155:382–92.e1.
Article8. Kikuchi E, Yamazaki K, Torigoe T, et al. HLA class I antigen expression is associated with a favorable prognosis in early stage nonsmall cell lung cancer. Cancer Sci. 2007; 98:1424–30.
Article9. Ramnath N, Tan D, Li Q, et al. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006; 55:891–9.
Article10. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.11. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015; 10:1243–60.12. Kim H, Kwon HJ, Park SY, Park Y, Park E, Chung JH. Clinicopathological analysis and prognostic significance of programmed cell death-ligand 1 protein and mRNA expression in non-small cell lung cancer. PLoS One. 2018; 13:e0198634.
Article13. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–50.
Article14. Perea F, Sánchez-Palencia A, Gómez-Morales M, et al. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget. 2018; 9:4120–33.
Article15. Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011; 132:315–25.
Article16. Ljunggren HG, Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990; 11:237–44.
Article17. Imai D, Yoshizumi T, Okano S, et al. The prognostic impact of programmed cell death ligand 1 and human leukocyte antigen class I in pancreatic cancer. Cancer Med. 2017; 6:1614–26.
Article18. Mizukami Y, Kono K, Maruyama T, et al. Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2008; 99:1462–7.
Article19. Umemoto Y, Okano S, Matsumoto Y, et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol. 2015; 50:65–75.
Article20. Menon AG, Morreau H, Tollenaar RA, et al. Down-regulation of HLA-A expression correlates with a better prognosis in colorectal cancer patients. Lab Invest. 2002; 82:1725–33.
Article21. Ueda Y, Ishikawa K, Shiraishi N, Yokoyama S, Kitano S. Clinical significance of HLA class I heavy chain expression in patients with gastric cancer. J Surg Oncol. 2008; 97:451–5.
Article22. Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017; 7:10255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiotherapy and immune checkpoint blockades: a snapshot in 2016
- A Case of Anti-programmed Cell Death Ligand 1 and Anti-transforming Growth Factor Beta Antibody-associated Keratoacanthoma
- Cancer Immunotherapy Related Endocrine Adverse Effects
- Immune Evasion of G-CSF and GM-CSF in Lung Cancer
- An update on immunotherapy with PD-1 and PD-L1 blockade