Cancer Res Treat.
2019 Apr;51(2):438-450. 10.4143/crt.2018.040.
Preclinical Efficacy of [Vâ´Qâµ]dDAVP, a Second Generation Vasopressin Analog, on Metastatic Spread and Tumor-Associated Angiogenesis in Colorectal Cancer
- Affiliations
-
- 1Laboratory of Molecular Oncology, Science and Technology Department, National University of Quilmes, Buenos Aires, Argentina. juan.garona@unq.edu.ar
- KMID: 2464391
- DOI: http://doi.org/10.4143/crt.2018.040
Abstract
- PURPOSE
Control of metastatic spread of colorectal cancer (CRC) remains as a major therapeutic challenge. [V4 Q5 ]dDAVP is a vasopressin peptide analog with previously reported anticancer activity against carcinoma tumors. By acting as a selective agonist of arginine vasopressin type 2 membrane receptor (AVPR2) present in endothelial and tumor cells, [Vâ´Qâµ]dDAVP is able to impair tumor aggressiveness and distant spread. Our aim was to evaluate the potential therapeutic benefits of [Vâ´Qâµ]dDAVP on highly aggressive CRC disease using experimental models with translational relevance.
MATERIALS AND METHODS
Murine CT-26 and human Colo-205 AVPR2-expressing CRC cell lines were used to test the preclinical efficacy of [Vâ´Qâµ]dDAVP, both in vitro and in vivo.
RESULTS
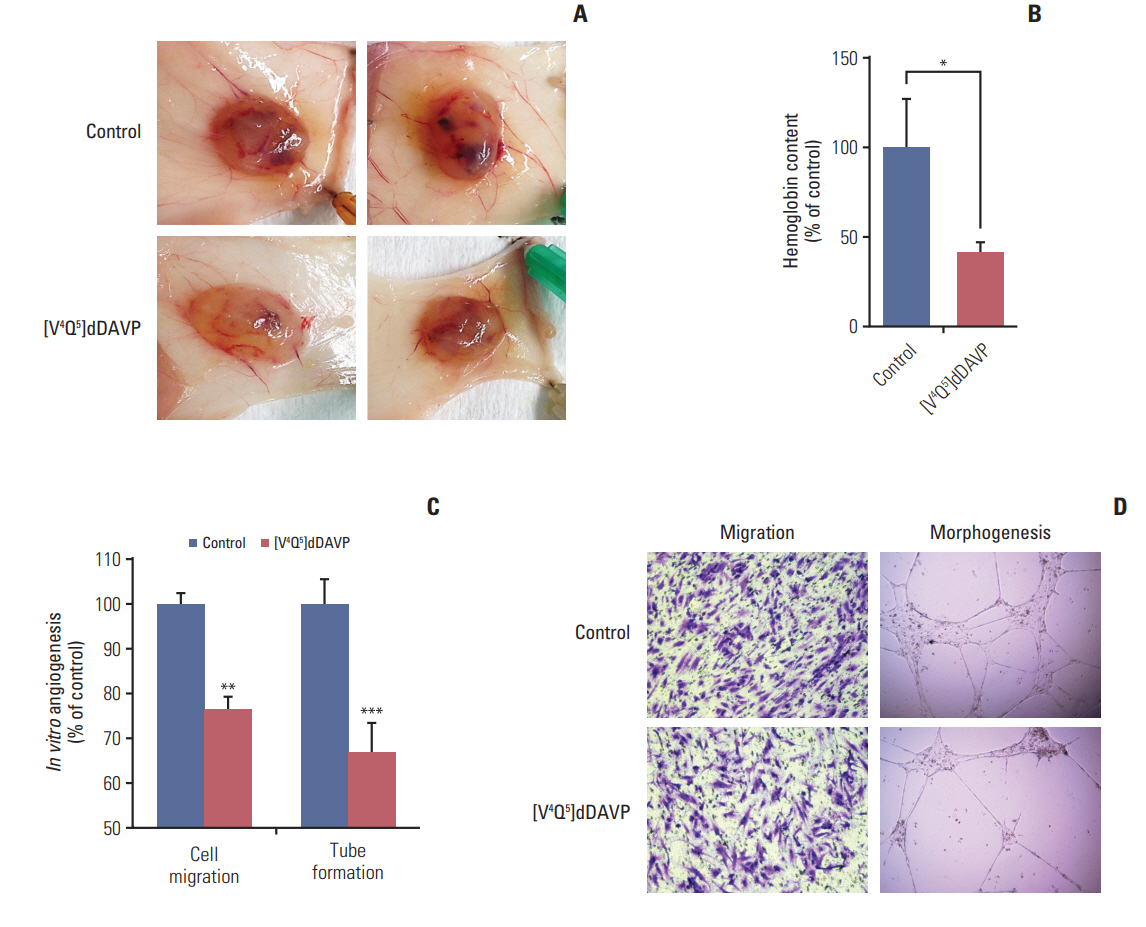
In syngeneic mice surgically implanted with CT-26 cells in the spleen, sustained intravenous treatment with [Vâ´Qâµ]dDAVP (0.3 µg/kg) dramatically impaired metastatic progression to liver without overt signs of toxicity, and also reduced experimental lung colonization. The compound inhibited in vivo angiogenesis driven by Colo-205 cells in athymic mice, as well as in vitro endothelial cell migration and capillary tube formation. [Vâ´Qâµ]dDAVP exerted AVPR2-dependent cytostatic activity in vitro (ICâ‚…â‚€ 1.08 µM) and addition to 5-fluorouracil resulted in synergistic antiproliferative effects both in CT-26 and Colo-205 cells.
CONCLUSION
The present preclinical study establishes for the first time the efficacy of [Vâ´Qâµ]dDAVP on CRC. These encouraging results suggest that the novel second generation vasopressin analog could be used for the management of aggressive CRC as an adjuvant agent during surgery or to complement standard chemotherapy, limiting tumor angiogenesis and metastasis and thus protecting the patient from CRC recurrence.
MeSH Terms
-
Animals
Arginine Vasopressin
Capillaries
Cell Line
Colon
Colorectal Neoplasms*
Complement System Proteins
Drug Therapy
Endothelial Cells
Fluorouracil
Humans
In Vitro Techniques
Liver
Lung
Membranes
Mice
Mice, Nude
Models, Theoretical
Neoplasm Metastasis
Recurrence
Robenidine
Spleen
Vasopressins*
Arginine Vasopressin
Complement System Proteins
Fluorouracil
Robenidine
Vasopressins
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Alonso DF, Skilton G, Farias EF, Bal de Kier Joffe E, Gomez DE. Antimetastatic effect of desmopressin in a mouse mammary tumor model. Breast Cancer Res Treat. 1999; 57:271–5.
Article3. Ripoll GV, Garona J, Pifano M, Farina HG, Gomez DE, Alonso DF. Reduction of tumor angiogenesis induced by desmopressin in a breast cancer model. Breast Cancer Res Treat. 2013; 142:9–18.
Article4. Kaufmann JE, Oksche A, Wollheim CB, Gunther G, Rosenthal W, Vischer UM. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000; 106:107–16.
Article5. Pifano M, Garona J, Capobianco CS, Gonzalez N, Alonso DF, Ripoll GV. Peptide agonists of vasopressin V2 receptor reduce expression of neuroendocrine markers and tumor growth in human lung and prostate tumor cells. Front Oncol. 2017; 7:11.
Article6. Ripoll GV, Garona J, Hermo GA, Gomez DE, Alonso DF. Effects of the synthetic vasopressin analog desmopressin in a mouse model of colon cancer. Anticancer Res. 2010; 30:5049–54.7. Mochizuki S, Soejima K, Shimoda M, Abe H, Sasaki A, Okano HJ, et al. Effect of ADAM28 on carcinoma cell metastasis by cleavage of von Willebrand factor. J Natl Cancer Inst. 2012; 104:906–22.
Article8. Terraube V, Marx I, Denis CV. Role of von Willebrand factor in tumor metastasis. Thromb Res. 2007; 120 Suppl 2:S64–70.
Article9. Kawecki C, Lenting PJ, Denis CV. von Willebrand factor and inflammation. J Thromb Haemost. 2017; 15:1285–94.
Article10. Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011; 117:1071–80.
Article11. Weinberg RS, Grecco MO, Ferro GS, Seigelshifer DJ, Perroni NV, Terrier FJ, et al. A phase II dose-escalation trial of perioperative desmopressin (1-desamino-8-d-arginine vasopressin) in breast cancer patients. Springerplus. 2015; 4:428.
Article12. Czaplewski C, Kazmierkiewicz R, Ciarkowski J. Molecular modeling of the human vasopressin V2 receptor/agonist complex. J Comput Aided Mol Des. 1998; 12:275–87.13. Kowalczyk W, Prahl A, Derdowska I, Sobolewski D, Olejnik J, Zabrocki J, et al. Analogues of neurohypophyseal hormones, oxytocin and arginine vasopressin, conformationally restricted in the N-terminal part of the molecule. J Med Chem. 2006; 49:2016–21.
Article14. Pastrian MB, Guzman F, Garona J, Pifano M, Ripoll GV, Cascone O, et al. Structure-activity relationship of 1-desamino-8-D-arginine vasopressin as an antiproliferative agent on human vasopressin V2 receptor-expressing cancer cells. Mol Med Rep. 2014; 9:2568–72.
Article15. Garona J, Pifano M, Orlando UD, Pastrian MB, Iannucci NB, Ortega HH, et al. The novel desmopressin analogue [V4Q5]dDAVP inhibits angiogenesis, tumour growth and metastases in vasopressin type 2 receptor-expressing breast cancer models. Int J Oncol. 2015; 46:2335–45.16. Iannucci NB, Ripoll GV, Garona J, Cascone O, Ciccia GN, Gomez DE, et al. Antiproliferative effect of 1-deamino-8-D-arginine vasopressin analogs on human breast cancer cells. Future Med Chem. 2011; 3:1987–93.
Article17. Monstein HJ, Truedsson M, Ryberg A, Ohlsson B. Vasopressin receptor mRNA expression in the human gastrointestinal tract. Eur Surg Res. 2008; 40:34–40.
Article18. Marques I, Araujo A, de Mello RA. Anti-angiogenic therapies for metastatic colorectal cancer: current and future perspectives. World J Gastroenterol. 2013; 19:7955–71.
Article19. Castle JC, Loewer M, Boegel S, de Graaf J, Bender C, Tadmor AD, et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics. 2014; 15:190.
Article20. Khan S, Cameron S, Blaschke M, Moriconi F, Naz N, Amanzada A, et al. Differential gene expression of chemokines in KRAS and BRAF mutated colorectal cell lines: role of cytokines. World J Gastroenterol. 2014; 20:2979–94.21. Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016; 6:29765.
Article22. Kim KY, Kim NK, Cha IH, Ahn JB, Choi JS, Choi GH, et al. Novel methods for clinical risk stratification in patients with colorectal liver metastases. Cancer Res Treat. 2015; 47:242–50.
Article23. Rmali KA, Puntis MC, Jiang WG. Tumour-associated angiogenesis in human colorectal cancer. Colorectal Dis. 2007; 9:3–14.
Article24. Ripoll G, Iannucci N, Giron S, Cascone O, Gomez D, Alonso D. Angiostatic activity of 1-deamino-8-D-arginine vasopressin and novel peptide analogues in breast cancer cells. Cancer Res. 2008; 68(9 Suppl):295.25. Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015; 12:213–26.
Article26. Hirai T, Matsumoto H, Kubota H, Yamaguchi Y. Regulating surgical oncotaxis to improve the outcomes in cancer patients. Surg Today. 2014; 44:804–11.
Article27. Pifano M, Garona J, Sobol NT, Alberto M, Alonso DF, Ripoll GV. Search of vasopressin analogs with antiproliferative activity on small-cell lung cancer: drug design based on two different approaches. Future Med Chem. 2018; 10:879–94.
Article28. McEwan DG, Brunton VG, Baillie GS, Leslie NR, Houslay MD, Frame MC. Chemoresistant KM12C colon cancer cells are addicted to low cyclic AMP levels in a phosphodiesterase 4-regulated compartment via effects on phosphoinositide 3-kinase. Cancer Res. 2007; 67:5248–57.
Article29. Loffler I, Grun M, Bohmer FD, Rubio I. Role of cAMP in the promotion of colorectal cancer cell growth by prostaglandin E2. BMC Cancer. 2008; 8:380.
Article30. Hopfner M, Maaser K, Barthel B, von Lampe B, Hanski C, Riecken EO, et al. Growth inhibition and apoptosis induced by P2Y2 receptors in human colorectal carcinoma cells: involvement of intracellular calcium and cyclic adenosine monophosphate. Int J Colorectal Dis. 2001; 16:154–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sick Sinus Syndrome Following Severe Hyponatremia Associated with Desmopressin Therapy
- Correlation between expression of matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9 (MMP-9) and angiogenesis in colorectal adenocarcinoma
- Current Status of Chemotherapy in Colorectal Cancer: Updated Treatment Strategies
- Correlation between Tumor Angiogenesis (Microvessel Density), Metastasis and Tumor Cell Proliferation in Colorectal Carcinomas
- Expression of Vascular Endothelial Growth Factor (VEGF) and p53 in Colorectal Cancer