J Korean Assoc Oral Maxillofac Surg.
2019 Oct;45(5):267-275. 10.5125/jkaoms.2019.45.5.267.
Role of immunoreactive patterns of lymph nodes in neck dissection cases of oral squamous cell carcinoma: a clinical and histopathological study
- Affiliations
-
- 1Department of Oral Pathology and Microbiology, KLE Academy of Higher Education and Research (KLE University), KLE VK Institute of Dental Sciences and Hospital, Belagavi, India. harshadacb9@gmail.com
- 2Department of Oral and Maxillofacial Surgery, KLE Academy of Higher Education and Research (KLE University), KLE VK Institute of Dental Sciences and Hospital, Belagavi, India.
- KMID: 2461479
- DOI: http://doi.org/10.5125/jkaoms.2019.45.5.267
Abstract
OBJECTIVES
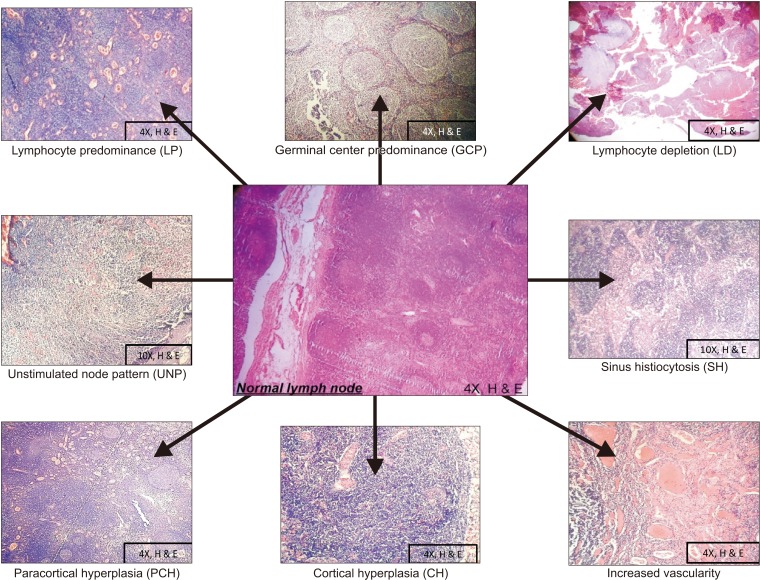
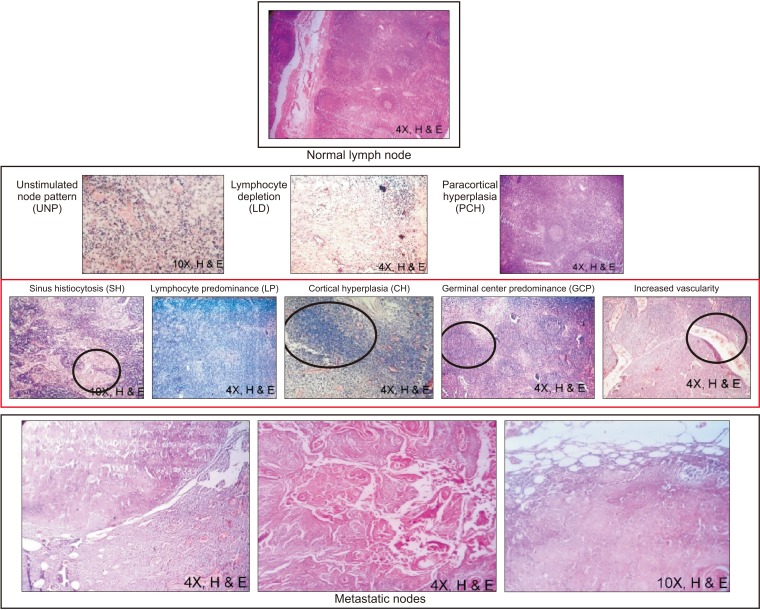
Metastasis in oral squamous cell carcinoma (OSCC) can occur in a variety of ways, and draining lymphatics and lymph nodes serve as a common route. Prior to metastasis, lymph nodes elicit an immune response to either wall off or create a favorable environment for homing of tumor cells. This immune response to tumor stimuli is visualized by recognizing various immunoreactive patterns exhibited by the lymph node. The present study aims to evaluate the role of immuno-morphologic patterns of the lymph node in neck dissection for cases of OSCC.
MATERIALS AND METHODS
Our retrospective study included 50 neck dissection cases of OSCC and a total of 1,078 lymph nodes. The grades of primary tumors with eight different immunoreactive patterns were compared. Vascularity and metastasis in lymph nodes were also evaluated.
RESULTS
The lymphocyte predominant pattern was the most common immunoreactive pattern found in 396 of 1,078 lymph nodes. Patterns of lymphocyte predominant (P=0.0005), sinus histiocytosis (P=0.0500), paracortical hyperplasia (P=0.0001), cortical hyperplasia (P=0.0001), and increased vascularity (P=0.0190) were significantly associated with tumor grade.
CONCLUSION
The present study adds to the understanding of lymph node immunoreactivity patterns and their correlation with tumor grade. We recommend further study of lymph node patterns for all sentinel lymph node biopsies and routine neck dissections for OSCCs.
MeSH Terms
Figure
Reference
-
1. Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015; 8:11884–11894. PMID: 26617944.2. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009; 45:309–316. PMID: 18804401.
Article3. Bryne M. Prognostic value of various molecular and cellular features in oral squamous cell carcinomas: a review. J Oral Pathol Med. 1991; 20:413–420. PMID: 1804985.
Article4. Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006; 34:409–424. PMID: 17067937.
Article5. Cottier H, Turk J, Sobin L. A proposal for a standardized system of reporting human lymph node morphology in relation to immunological function. J Clin Pathol. 1973; 26:317–331. PMID: 4123530.
Article6. Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987; 95:229–249. PMID: 3299675.
Article7. Shingaki S, Suzuki I, Nakajima T, Kawasaki T. Evaluation of histopathologic parameters in predicting cervical lymph node metastasis of oral and oropharyngeal carcinomas. Oral Surg Oral Med Oral Pathol. 1988; 66:683–688. PMID: 3205557.
Article8. Jakobsson PA, Eneroth CM, Killander D, Moberger G, Mårtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973; 12:1–8. PMID: 4725642.
Article9. Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992; 166:375–381. PMID: 1517891.
Article10. Klijanienko J, el-Naggar AK, de Braud F, Rodriguez-Peralto JL, Rodriguez R, Itzhaki M, et al. Tumor vascularization, mitotic index, histopathologic grade, and DNA ploidy in the assessment of 114 head and neck squamous cell carcinomas. Cancer. 1995; 75:1649–1656. PMID: 8826923.
Article11. Odell EW, Jani P, Sherriff M, Ahluwalia SM, Hibbert J, Levison DA, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. The importance of the pattern of invasion. Cancer. 1994; 74:789–794. PMID: 8039106.
Article12. Akhter M, Hossain S, Rahman QB, Molla MR. A study on histological grading of oral squamous cell carcinoma and its co-relationship with regional metastasis. J Oral Maxillofac Pathol. 2011; 15:168–176. PMID: 22529575.
Article13. Saldanha P. Morphological assessment of lymph nodes draining carcinoma. MGM J Med Sci. 2016; 3:190–197.
Article14. Tsakraklides V, Anastassiades OT, Kersey JH. Prognostic significance of regional lymph node histology in uterine cervical cancer. Cancer. 1973; 31:860–868. PMID: 4706051.
Article15. Schnitzer B. Reactive lymphoid hyperplasia. In : Jaffe ES, editor. Surgical pathology of the lymph nodes and related organs. 2nd ed. Philadelphia: W.B. Saunders;1995. p. 98–132.16. Stankiewicz C. Prognostic significance of lymph node reactivity in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol. 1994; 251:418–422. PMID: 7857630.
Article17. Malicka K. The assessment of defense function of lymph nodes on the basis of microscopical and clinical studies in laryngeal carcinoma. BTN Pr Wydz Nauk Przyr A. 1970; 14:5–29.18. Bennett SH, Futrell JW, Roth JA, Hoye RC, Ketcham AS. Prognostic significance of histologic host response in cancer of the larynx or hypopharynx. Cancer. 1971; 28:1255–1265. PMID: 5125672.
Article19. Silverberg SG, Chitale AR, Hind AD, Frazier AB, Levitt SH. Sinus histiocytosis and mammary carcinoma. Study of 366 radical mastectomies and an historical review. Cancer. 1970; 26:1177–1185. PMID: 5483650.
Article20. Kaufmann M, Wirth K, Scheurer J, Zimmermann A, Luscieti P, Stjernswärd J. Immunomorphological lymph node changes in patients with operable bronchogenic squamous cell carcinoma. Cancer. 1977; 39:2371–2377. PMID: 872035.
Article21. Bialek EJ, Jakubowski W, Szczepanik AB, Maryniak RK, Prochorec-Sobieszek M, Bilski R, et al. Vascular patterns in superficial lymphomatous lymph nodes: a detailed sonographic analysis. J Ultrasound. 2007; 10:128–134. PMID: 23396624.
Article22. Ahuja AT, Ying M, Ho SS, Metreweli C. Distribution of intranodal vessels in differentiating benign from metastatic neck nodes. Clin Radiol. 2001; 56:197–201. PMID: 11247696.
Article23. Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg. 1984; 6:938–947. PMID: 6724960.
Article24. Anderson AO, Anderson ND. Studies on the structure and permeability of the microvasculature in normal rat lymph nodes. Am J Pathol. 1975; 80:387–418. PMID: 1163637.25. Ioachim HL, Medeiros LJ. Reactive lymphoid hyperplasia. In : Ioachim HL, Medeiros LJ, editors. Ioachim's lymph node pathology. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2009. p. 171–180.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Elective Neck Dissection in Oral Squamous Cell Carcinoma
- Is it Necessary to Dissect Level I in Laryngeal and Hypopharyngeal Squamous Cell Carcinoma?
- Diagnostic accuracy of 18F-FDG in nodal negative oral squamous cell carcinoma
- Sentinel Lymph Node Biopsy in the Oral Cavity Cancer
- Is the Dissection of the Level IV Lymph Node Pads Necessary in the Elective Lateral Neck Dissection of N0 Supraglottic Squamous Cell Carcinoma?