Ann Lab Med.
2020 Mar;40(2):164-168. 10.3343/alm.2020.40.2.164.
Prevalence and Risk Factors for Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae Colonization in Intensive Care Units
- Affiliations
-
- 1Department of Laboratory Medicine, National Health Insurance Service, Ilsan Hospital, Goyang, Korea. yakim@nhimc.or.kr
- 2Department of Internal Medicine, National Health Insurance Service, Ilsan Hospital, Goyang, Korea. yspark@nhimc.or.kr
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2460914
- DOI: http://doi.org/10.3343/alm.2020.40.2.164
Abstract
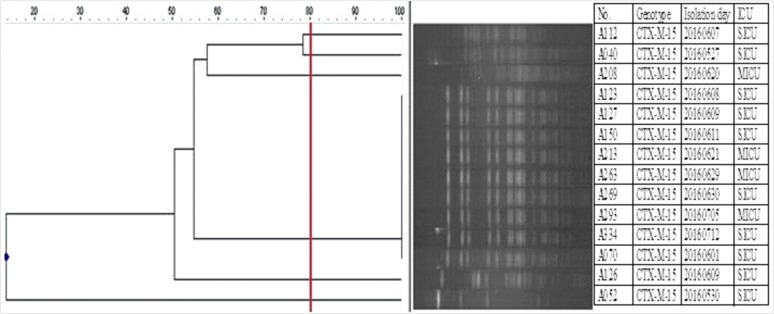
- Active surveillance culture (ASC) can help detect hidden reservoirs, but the routine use of ASC for extended spectrum β-lactamase-producing Enterobacteriaceae is controversial in an endemic situation. We aimed to determine the prevalence and risk factors of extended spectrum β-lactamase-producing Klebsiella pneumoniae (EBSL-Kpn) colonization among intensive care unit (ICU)-admitted patients. Prospective screening of ESBL-Kpn colonization was performed for ICU-admitted patients within 48 hours for two months. A perirectal swab sample was inoculated on MacConkey agar supplemented with 2 µg/mL ceftazidime. ESBL genotype was determined by PCR-sequencing, and clonal relatedness was evaluated by pulsed-field gel electrophoresis (PFGE). The risk factors of ESBL-Kpn colonization were evaluated. The ESBL-Kpn colonization rate among the 281 patients at ICU admission was 6.4% (18/281), and bla(CTX-M-15) was detected in all isolates. ESBL producers also showed resistance to fluoroquinolone (38.9%, 7/18). All isolates had the same ESBL genotype (bla(CTX-M-15)) and a highly clustered PFGE pattern, suggesting cross-transmission without a documented outbreak. In univariate analysis, the risk factor for ESBL-Kpn colonization over the control was the length of hospital stay (odds ratio=1.062; P=0.019). Routine use of ASC could help control endemic ESBL-Kpn for ICU patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Zahar JR, Blot S, Nordmann P, Martischang R, Timsit JF, Harbarth S, et al. Screening for intestinal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in critically ill patients: expected benefits and evidence-based controversies. Clin Infect Dis. 2019; 68:2125–2130. PMID: 30312366.2. Lee Y, Kim YA, Song W, Lee H, Lee HS, Jang SJ, et al. Recent trends in antimicrobial resistance in intensive care units in Korea. Korean J Nosocomial Infect Control. 2014; 19:29–36.3. Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 2018; 23:1800047.4. Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003; 24:362–386. PMID: 12785411.5. Thouverez M, Talon D, Bertrand X. Control of Enterobacteriaceae producing extended-spectrum beta-lactamase in intensive care units: rectal screening may not be needed in non-epidemic situations. Infect Control Hosp Epidemiol. 2004; 25:838–841. PMID: 15518025.6. CLSI. Performance standards for antimicrobial susceptibility testing. 26th ed. CLSI M100S. . Wayne, PA: Clinical and Laboratory Standards Institute;2016.7. Ryoo NH, Kim EC, Hong SG, Park YJ, Lee K, Bae IK, et al. Dissemination of SHV-12 and CTX-M-type extended-spectrum β-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae and emergence of GES-3 in Korea. J Antimicrob Chemother. 2005; 56:698–702. PMID: 16141280.8. Lee K, Cho SR, Lee CS, Chong Y, Kwon OH. Prevalence of extended broad-spectrum beta-lactamase in Escherichia coli and Klebsiella pneumonia. Korean J Infect Dis. 1994; 26:341–348.9. Park YS, Bae IK, Kim J, Jeong SH, Hwang SS, Seo YH, et al. Risk factors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli bacteremia. Yonsei Med J. 2014; 55:467–475. PMID: 24532519.10. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–797. PMID: 12435215.11. Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis. 2017; 65:208–215. PMID: 28369261.12. Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004; 39:219–226. PMID: 15307031.13. Martín-Loeches I, Diaz E, Vallés J. Risks for multidrug-resistant pathogens in the ICU. Curr Opin Crit Care. 2014; 20:516–524. PMID: 25188366.14. Starzyk-Łuszcz K, Zielonka TM, Jakubik J, Życińska K. Mortality due to nosocomial infection with Klebsiella pneumoniae ESBL+. Adv Exp Med Biol. 2017; 1022:19–26. PMID: 28456930.15. Gurieva T, Dautzenberg MJD, Gniadkowski M, Derde LPG, Bonten MJM, Bootsma MCJ. The transmissibility of antibiotic-resistant Enterobacteriaceae in intensive care units. Clin Infect Dis. 2018; 66:489–493. PMID: 29020273.16. Ko YJ, Moon HW, Hur M, Park CM, Cho SE, Yun YM. Fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in Korean community and hospital settings. Infection. 2013; 41:9–13. PMID: 22723075.17. Kim J, Lee JY, Kim SI, Song W, Kim JS, Jung S, et al. Rates of fecal transmission of extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacteriaceae among patients in intensive care units in Korea. Ann Lab Med. 2014; 34:20–25. PMID: 24422191.18. Kim D, Ahn JY, Lee CH, Jang SJ, Lee H, Yong D, et al. Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: analysis of Korean antimicrobial resistance monitoring system (KARMS) data from 2013 to 2015. Ann Lab Med. 2017; 37:231–239. PMID: 28224769.19. Jeong SH, Lee KM, Lee J, Bae IK, Kim JS, Kim HS, et al. Clonal and horizontal spread of the blaOXA-232 gene among Enterobacteriaceae in a Korean hospital. Diagn Microbiol Infect Dis. 2015; 82:70–72. PMID: 25702524.20. Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, et al. Carbapenemase-producing organisms: A global scourge. Clin Infect Dis. 2018; 66:1290–1297. PMID: 29165604.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Neonatal Osteomyelitis due to Extended Spectrum beta-lactamase Producing Klebsiella pneumoniae
- In Vitro Susceptibility of piperacillin/tazobactam Against extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae
- Successful Control of Extended-spectrum Beta-lactamase-producing Klebsiella pneumoniae Outbreak in a Neonatal Intensive Care Unit
- Ventriculitis Associated with Extended Spectrum Beta-Lactamase Producing Klebsiella pneumoniae after Acupuncture
- Evaluation of the Method to Screen Isolates of Extended-Spectrum -Lactamase-Producing Klebsiella pneumoniae and Escherichia coli Using Cefpodoxime Disk