Ann Surg Treat Res.
2019 Oct;97(4):184-193. 10.4174/astr.2019.97.4.184.
Which strategy is better for resectable synchronous liver metastasis from colorectal cancer, simultaneous surgery, or staged surgery? Multicenter retrospective analysis
- Affiliations
-
- 1Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. yslee@catholic.ac.kr
- 2Department of Surgery, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
- 3Department of Surgery, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 4Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Surgery, Chonnam National University Hwasun Hospital and Medical School, Gwangju, Korea.
- 6Department of Surgery, Korea University Anam Hospital, Seoul, Korea.
- 7Department of Surgery, Dong-A University College of Medicine, Busan, Korea.
- 8Department of Surgery, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 9Department of Surgery, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- KMID: 2459316
- DOI: http://doi.org/10.4174/astr.2019.97.4.184
Abstract
- PURPOSE
The optimal treatment for synchronous liver metastasis (LM) from colorectal cancer (CRC) depends on various factors. The present study was intended to investigate the oncologic outcome according to the time of resection of metastatic lesions.
METHODS
Data from patients who underwent treatment with curative intent for primary CRC and synchronous LM between 2004 and 2009 from 9 university hospitals in Korea were collected retrospectively. One hundred forty-three patients underwent simultaneous resection for primary CRC and synchronous LM (simultaneous surgery group), and 65 patients were treated by 2-stage operation (staged surgery group).
RESULTS
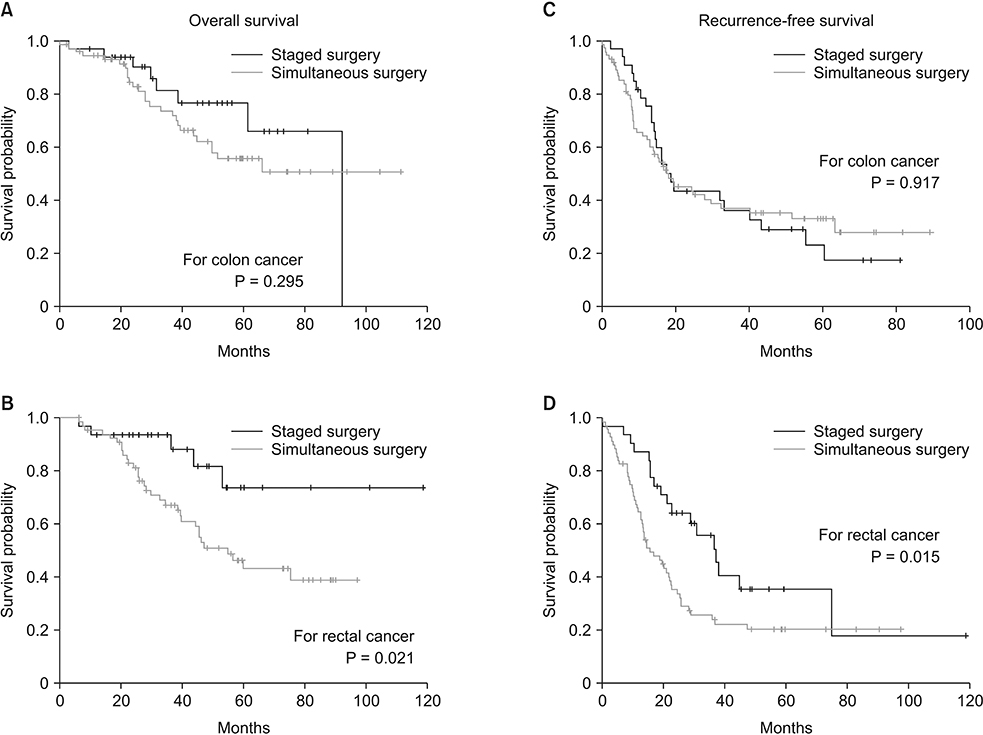
The mean follow-up length was 41.2 ± 24.6 months. In the extent of resection for hepatic metastasis, major hepatectomy was more frequently performed in staged surgery group (33.8% vs. 8.4%, P < 0.001). The rate of severe complications of Clavien-Dindo classification grade III or more was not significantly different between the 2 groups. The 3-year overall survival (OS) rate was 85.0% in staged surgery group and 69.4% in simultaneous surgery group (P = 0.013), and the 3-year recurrence-free survival (RFS) rate was 46.4% in staged surgery group and 30.2% in simultaneous surgery group (P = 0.143). In subgroup analysis based on the location of primary CRC, the benefit of staged surgery for OS and RFS was clearly shown in rectal cancer (P = 0.021 and P = 0.015).
CONCLUSION
Based on our results, staged surgery with or without neoadjuvant chemotherapy should be considered for resectable synchronous LM from CRC, especially in rectal cancer, as a safe and fairly promising option.
MeSH Terms
Figure
Cited by 1 articles
-
Can the presence of KRAS mutations guide the type of liver resection during simultaneous resection of colorectal liver metastasis?
Munseok Choi, Dai Hoon Han, Jin Sub Choi, Gi Hong Choi
Ann Hepatobiliary Pancreat Surg. 2022;26(2):125-132. doi: 10.14701/ahbps.21-127.
Reference
-
1. Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat. 2012; 44:219–226.
Article2. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007; 25:4575–4580.
Article3. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25 Suppl 3:iii1–iii9.
Article4. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008; 247:125–135.5. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009; 27:3677–3683.
Article6. Martin RC 2nd, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009; 208:842–850.
Article7. Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004; 240:1052–1061.8. Wang Y, Wang ZQ, Wang FH, Yuan YF, Li BK, Ding PR, et al. The role of adjuvant chemotherapy for colorectal l iver metastasectomy after pre-operative chemotherapy: is the treatment worthwhile? J Cancer. 2017; 8:1179–1186.9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.10. Martin R, Paty P, Fong Y, Grace A, Cohen A, DeMatteo R, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003; 197:233–241.
Article11. Yin Z, Liu C, Chen Y, Bai Y, Shang C, Yin R, et al. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): Simultaneous or delayed? Hepatology. 2013; 57:2346–2357.
Article12. Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000; 231:743–751.
Article13. Nakajima K, Takahashi S, Saito N, Kotaka M, Konishi M, Gotohda N, et al. Predictive factors for anastomotic leakage after simultaneous resection of synchronous colorectal liver metastasis. J Gastrointest Surg. 2012; 16:821–827.
Article14. Slesser AA, Simillis C, Goldin R, Brown G, Mudan S, Tekkis PP. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg Oncol. 2013; 22:36–47.
Article15. Minagawa M, Yamamoto J, Miwa S, Sakamoto Y, Kokudo N, Kosuge T, et al. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006; 141:1006–1012.
Article16. Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006; 93:872–878.
Article17. Mayo SC, Pulitano C, Marques H, Lamelas J, Wolfgang CL, de Saussure W, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg. 2013; 216:707–716.
Article18. Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015; 41:729–741.
Article19. Yoshidome H, Kimura F, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, et al. Interval period tumor progression: does delayed hepatectomy detect occult metastases in synchronous colorectal liver metastases. J Gastrointest Surg. 2008; 12:1391–1398.
Article20. Araujo R, Gonen M, Allen P, Blumgart L, DeMatteo R, Fong Y, et al. Comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic colorectal cancer. Ann Surg Oncol. 2013; 20:4312–4321.
Article21. Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: friend or foe. Ann Surg. 2012; 255:237–247.22. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008; 371:1007–1016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aggressive surgical resection for concomitant liver and lung metastasis in colorectal cancer
- Simultaneous resection of synchronous colorectal liver metastasis: Feasibility and development of a prediction model
- Primary tumor sidedness is not prognostic factor in resectable colorectal cancer liver metastasis: a retrospective observational cohort study
- Surgical resection of synchronous and metachronous lung and liver metastases of colorectal cancers
- Simultaneous Laparoscopy-Assisted Resection for Colorectal Cancer and Metastases