Ann Surg Treat Res.
2019 Oct;97(4):159-167. 10.4174/astr.2019.97.4.159.
Antioxidant action of hypoxic conditioned media from adipose-derived stem cells in the hepatic injury of expressing higher reactive oxygen species
- Affiliations
-
- 1Catholic Central Laboratory of Surgery, Institute of Biomedical Industry, College of Medicine, The Catholic University of Korea, Seoul, Korea. sayjunekim@gmail.com
- 2Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Surgery, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2459313
- DOI: http://doi.org/10.4174/astr.2019.97.4.159
Abstract
- PURPOSE
Almost all liver diseases are known to be accompanied by increased levels of reactive oxygen species (ROS), regardless of the cause of the liver disorder. However, little is known about the role of hypoxic conditioned media (HCM) in the view of pro-oxidative/antioxidative balance.
METHODS
Normoxic conditioned media (NCM) and HCM were obtained after culturing adipose-derived stem cells in 20% Oâ‚‚ or 1% Oâ‚‚ for 24 hours, respectively. Their effects on the expression of various markers reflecting pro-oxidative/antioxidative balance were investigated in both in vitro (thioacetamide-treated AML12 cells) and in vivo (partially hepatectomized mice) models of liver injury, respectively.
RESULTS
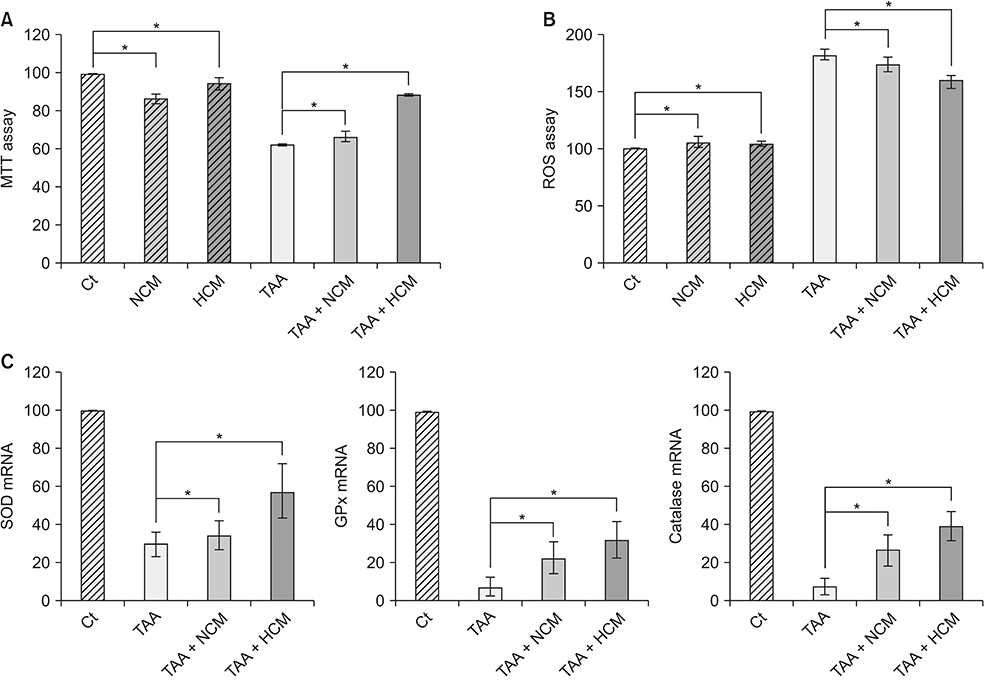
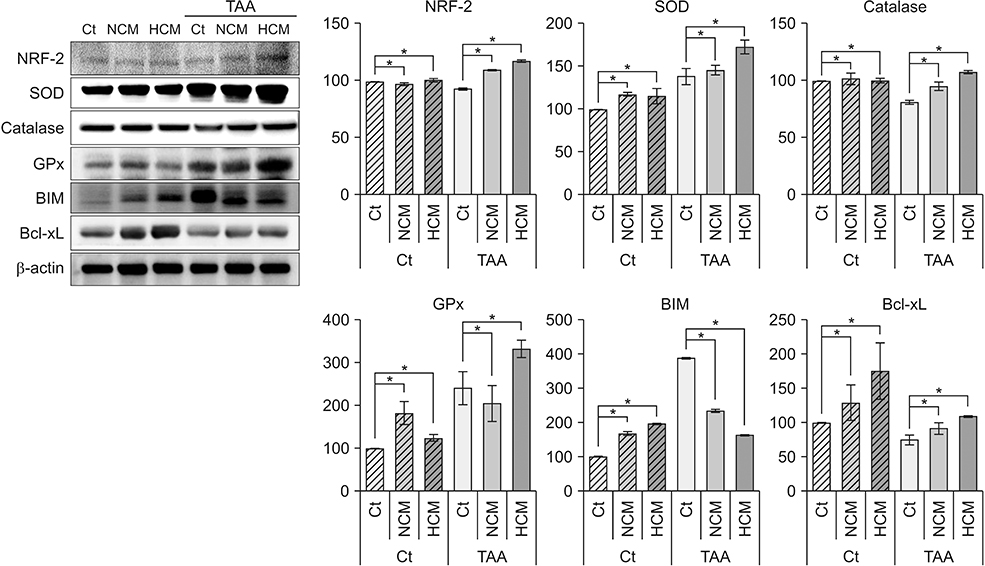
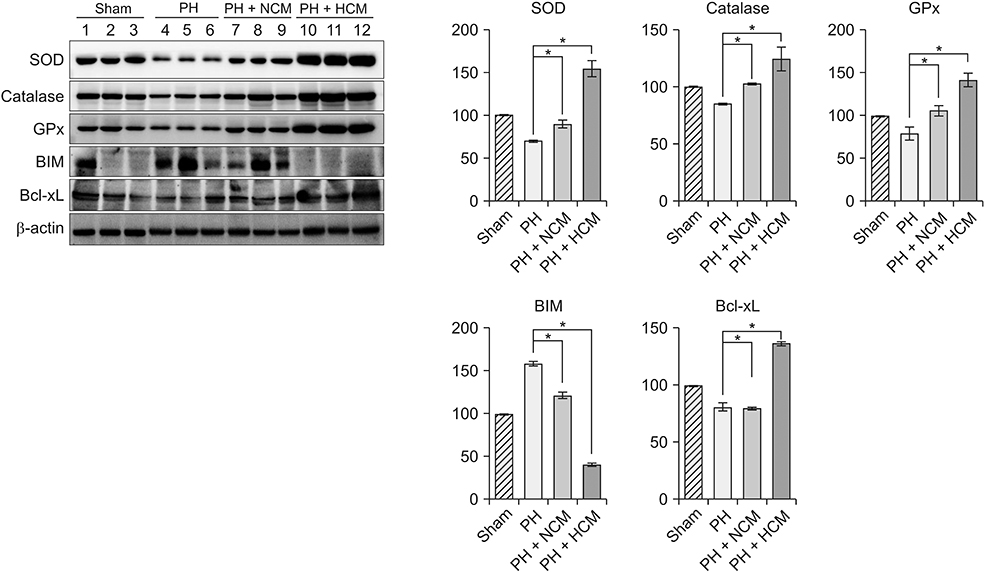
HCM treatment induced the higher expression of antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and catalase than did NCM in the in vitro model of liver injury. We also found that HCM increased the expression of nuclear factor erythroid 2-related factor (NRF2). The in vivo models of liver injury consistently validated the phenomenon of upregulated expression of antioxidant enzymes by HCM.
CONCLUSION
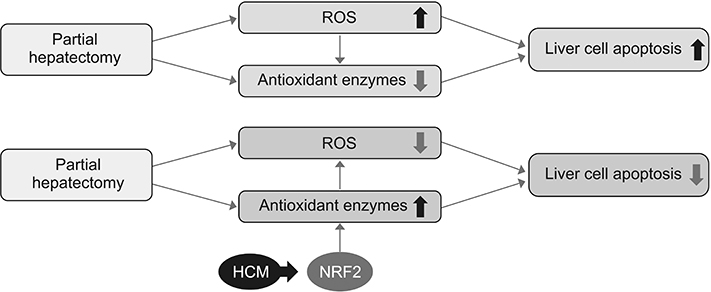
We thus could conclude that HCM provides protection against ROS-related toxicity by increasing the expression of antioxidant enzymes, in part by releasing NRF2 in the injured liver.
Keyword
MeSH Terms
Figure
Reference
-
1. Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012; 3:359.
Article2. Fink T, Abildtrup L, Fogd K, Abdallah BM, Kassem M, Ebbesen P, et al. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells. 2004; 22:1346–1355.
Article3. Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic conditioned medium from human adipose-derived stem cells promotes mouse liver regeneration through JAK/STAT3 signaling. Stem Cells Transl Med. 2016; 5:816–825.
Article4. Lee SC, Kim KH, Kim OH, Lee SK, Hong HE, Won SS, et al. Determination of optimized oxygen partial pressure to maximize the liver regenerative potential of the secretome obtained from adipose-derived stem cells. Stem Cell Res Ther. 2017; 8:181.
Article5. Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013; 37:551–560.
Article6. Yue Y, Zhang P, Liu D, Yang JF, Nie C, Yang D. Hypoxia preconditioning enhances the viability of ADSCs to increase the survival rate of ischemic skin flaps in rats. Aesthetic Plast Surg. 2013; 37:159–170.
Article7. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5:9–19.
Article8. Cichoz-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014; 20:8082–8091.9. Lee SM, Lee SC, Kim SJ. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res. 2014; 188:280–289.
Article10. Fouraschen SM, Pan Q, de Ruiter PE, Farid WR, Kazemier G, Kwekkeboom J, et al. Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 2012; 21:2410–2419.
Article11. Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003; 16:99–102.
Article12. Choi CW, Park EC, Yun SH, Lee SY, Lee YG, Hong Y, et al. Proteomic characterization of the outer membrane vesicle of Pseudomonas putida KT2440. J Proteome Res. 2014; 13:4298–4309.
Article13. Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondria sentencing about cellular life and death: a matter of oxidative stress. Curr Pharm Des. 2011; 17:4047–4060.
Article14. Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008; 49:133–142.
Article15. Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013; 10:301–312.
Article16. Schonthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol. 2013; 85:653–666.17. Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990; 87:1620–1624.
Article18. Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004; 101:4003–4008.
Article19. Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010; 29:248–253.
Article20. Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990; 346:281–284.21. Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-transforming growth factor-interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007; 282:35887–35898.22. Tufvesson E, Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002; 530:124–128.23. Alan C, Kocoglu H, Altintas R, Alici B, Resit Ersay A. Protective effect of decorin on acute ischaemia-reperfusion injury in the rat kidney. Arch Med Sci. 2011; 7:211–216.
Article24. Ozay R, Turkoglu E, Gurer B, Dolgun H, Evirgen O, Erguder BI, et al. Does decorin protect neuronal tissue via its antioxidant and antiinflammatory activity from traumatic brain injury? An experimental study. World Neurosurg. 2017; 97:407–415.25. Ma R, Chen J, Li Z, Tang J, Wang Y, Cai X. Decorin accelerates the liver regeneration after partial hepatectomy in fibrotic mice. Chin Med J (Engl). 2014; 127:2679–2685.26. Carlyle BC, Trombetta BA, Arnold SE. Proteomic approaches for the discovery of biofluid biomarkers of neurodegenerative dementias. Proteomes. 2018; 6(3):E32. DOI: 10.3390/proteomes6030032.
Article27. Li X, Wang W, Chen J. Recent progress in mass spectrometry proteomics for biomedical research. Sci China Life Sci. 2017; 60:1093–1113.
Article28. Tsuchida S, Satoh M, Takiwaki M, Nomura F. Current status of proteomic technologies for discovering and identifying gingival crevicular fluid biomarkers for periodontal disease. Int J Mol Sci. 2018; 20:E86.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Reactive Oxygen Species in the Adipogenesis of Adipose-derived Stem Cells
- Reactive Oxygen Species and Cancer
- Liver Regenerating Potential of the Secretome Obtained from Adipose-derived Stem Cells Cultured under the Hypoxic Environment
- L-carnitine Effectively Induces hTERT Gene Expression of Human Adipose Tissue-derived Mesenchymal Stem Cells Obtained from the Aged Subjects
- Effect of Dipyridamole on the Reactive Oxygen Species and Oxidative Stress in Trabecular Meshwork Cells