J Lipid Atheroscler.
2019 Sep;8(2):144-151. 10.12997/jla.2019.8.2.144.
Diabetes-related Amylin Dyshomeostasis: a Contributing Factor to Cerebrovascular Pathology and Dementia
- Affiliations
-
- 1Departments of Pharmacology and Nutritional Sciences, and Neurology, University of Kentucky, Lexington, KY, USA. f.despa@uky.edu
- KMID: 2458379
- DOI: http://doi.org/10.12997/jla.2019.8.2.144
Abstract
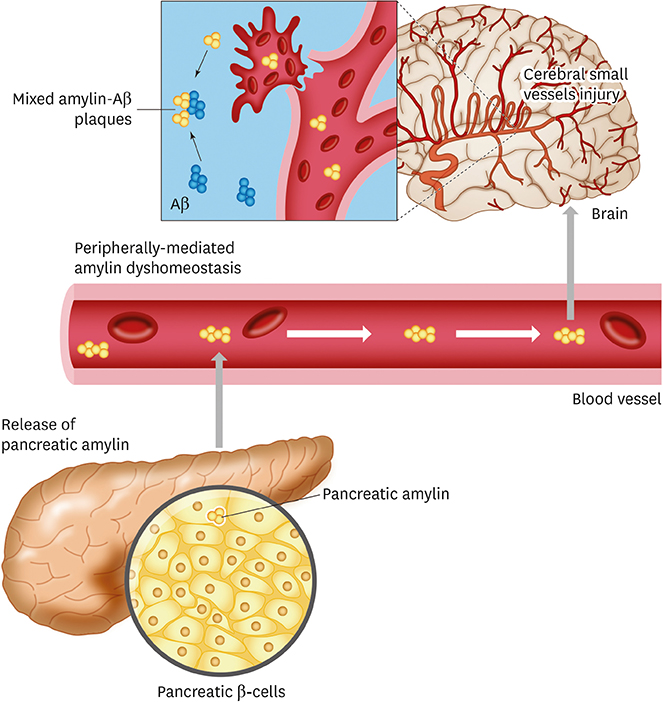
- Type 2 diabetes (T2D) increases the risk for cerebrovascular disease (CVD) and dementia. The underlying molecular mechanisms remain elusive, which hampers the development of treatment or/and effective prevention strategies. Recent studies suggest that dyshomeostasis of amylin, a satiety hormone that forms pancreatic amyloid in patients with T2D, promotes accumulation of amylin in cerebral small blood vessels and interaction with Alzheimer's disease (AD) pathology. Overexpression of human amylin in rodents (rodent amylin does not form amyloid) leads to late-life onset T2D and neurologic deficits. In this Review, we discuss clinical evidence of amylin pathology in CVD and AD and identify critical characteristics of animal models that could help to better understand molecular mechanisms underlying the increased risk of CVD and AD in patients with prediabetes or T2D.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cognitive Dysfunction and Diabetes
Chan-Hee Jung, Ji-Oh Mok
J Korean Diabetes. 2022;23(3):165-177. doi: 10.4093/jkd.2022.23.3.165.
Reference
-
1. Alzheimer's Association. 2019 Alzhiemer's disease facts and figures. Alzheimers Dement. 2019; 15:321–387.2. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013; 80:844–866.
Article3. Tong X, Yang Q, Ritchey MD, George MG, Jackson SL, Gillespie C, et al. The burden of cerebrovascular disease in the United States. Prev Chronic Dis. 2019; 16:E52.
Article4. Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014; 161:785–793.
Article5. Okereke OI, Kang JH, Cook NR, Gaziano JM, Manson JE, Buring JE, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008; 56:1028–1036.
Article6. Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999; 53:1937–1942.
Article7. Peila R, Rodriguez BL, Launer LJ. Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002; 51:1256–1262.
Article8. Geijselaers SL, Sep SJ, Stehouwer CD, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015; 3:75–89.
Article9. Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013; 369:1863–1864.
Article10. Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009; 32:221–226.
Article11. Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011; 10:969–977.
Article12. Feinkohl I, Price JF, Strachan MW, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther. 2015; 7:46.
Article13. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011; 91:795–826.
Article14. Ly H, Verma N, Wu F, Liu M, Saatman KE, Nelson PT, et al. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol. 2017; 82:208–222.
Article15. Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: a second amyloid in Alzheimer disease? Ann Neurol. 2013; 74:517–526.
Article16. Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015; 185:834–846.17. Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life Sci. 1995; 57:1993–2001.
Article18. Beaumont K, Kenney MA, Young AA, Rink TJ. High affinity amylin binding sites in rat brain. Mol Pharmacol. 1993; 44:493–497.19. Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci. 2006; 361:1219–1235.
Article20. Westermark P. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci. 1972; 77:91–94.21. Johnson KH, O'Brien TD, Betsholtz C, Westermark P. Islet amyloid, islet-amyloid polypeptide, and diabetes mellitus. N Engl J Med. 1989; 321:513–518.
Article22. Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988; 9:151–159.23. Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011; 178:2632–2640.
Article24. Gong W, Liu ZH, Zeng CH, Peng A, Chen HP, Zhou H, et al. Amylin deposition in the kidney of patients with diabetic nephropathy. Kidney Int. 2007; 72:213–218.
Article25. Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, et al. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats. Circ Res. 2012; 110:598–608.
Article26. Fawver JN, Ghiwot Y, Koola C, Carrera W, Rodriguez-Rivera J, Hernandez C, et al. Islet amyloid polypeptide (IAPP): a second amyloid in Alzheimer's disease. Curr Alzheimer Res. 2014; 11:928–940.27. Schultz N, Byman E, Fex M, Wennström M. Amylin alters human brain pericyte viability and NG2 expression. J Cereb Blood Flow Metab. 2017; 37:1470–1482.
Article28. Verma N, Ly H, Liu M, Chen J, Zhu H, Chow M, et al. Intraneuronal amylin deposition, peroxidative membrane injury and increased IL-1β synthesis in brains of Alzheimer's disease patients with type-2 diabetes and in diabetic HIP rats. J Alzheimers Dis. 2016; 53:259–272.
Article29. Schultz N, Byman E, Wennström M; Netherlands Brain Bank, Wennström M. Levels of retinal IAPP are altered in Alzheimer's disease patients and correlate with vascular changes and hippocampal IAPP levels. Neurobiol Aging. 2018; 69:94–101.
Article30. Moreno-Gonzalez I, Edwards G III, Salvadores N, Shahnawaz M, Diaz-Espinoza R, Soto C. Molecular interaction between type 2 diabetes and Alzheimer's disease through cross-seeding of protein misfolding. Mol Psychiatry. 2017; 22:1327–1334.
Article31. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018; 14:591–604.
Article32. Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014; 2:246–255.
Article33. Roostaei T, Nazeri A, Felsky D, De Jager PL, Schneider JA, Pollock BG, et al. Genome-wide interaction study of brain beta-amyloid burden and cognitive impairment in Alzheimer's disease. Mol Psychiatry. 2017; 22:287–295.
Article34. Jhamandas JH, MacTavish D. Antagonist of the amylin receptor blocks β-amyloid toxicity in rat cholinergic basal forebrain neurons. J Neurosci. 2004; 24:5579–5584.
Article35. Jhamandas JH, Li Z, Westaway D, Yang J, Jassar S, MacTavish D. Actions of β-amyloid protein on human neurons are expressed through the amylin receptor. Am J Pathol. 2011; 178:140–149.
Article36. Lim YA, Rhein V, Baysang G, Meier F, Poljak A, Raftery MJ, et al. Aβ and human amylin share a common toxicity pathway via mitochondrial dysfunction. Proteomics. 2010; 10:1621–1633.
Article37. Srodulski S, Sharma S, Bachstetter AB, Brelsfoard JM, Pascual C, Xie XS, et al. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014; 9:30.
Article38. Guénet JL. The mouse genome. Genome Res. 2005; 15:1729–1740.
Article39. King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012; 166:877–894.
Article40. Neha , Sodhi RK, Jaggi AS, Singh N. Animal models of dementia and cognitive dysfunction. Life Sci. 2014; 109:73–86.
Article41. Biessels GJ, Gispen WH. The impact of diabetes on cognition: what can be learned from rodent models? Neurobiol Aging. 2005; 26:Suppl 1. 36–41.
Article42. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006; 5:64–74.
Article43. Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014; 10:131–145.
Article44. Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006; 27:1420–1425.
Article45. Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006; 47:225–233.
Article