Investig Clin Urol.
2019 Sep;60(5):388-395. 10.4111/icu.2019.60.5.388.
Inflammation appears as high Prostate Imaging–Reporting and Data System scores on prostate magnetic resonance imaging (MRI) leading to false positive MRI fusion biopsy
- Affiliations
-
- 1Department of Urology, University of Texas Health San Antonio, San Antonio, TX, USA. liss@uthscsa.edu
- 2Department of Radiology, University of Texas Health San Antonio, San Antonio, TX, USA.
- 3Department of Pathology, University of Texas Health San Antonio, San Antonio, TX, USA.
- 4University of Texas Austin, College of Pharmacy, Austin, TX, USA.
- 5Mays Cancer Center UT Health San Antonio MD Anderson, San Antonio, TX, USA.
- KMID: 2455967
- DOI: http://doi.org/10.4111/icu.2019.60.5.388
Abstract
- PURPOSE
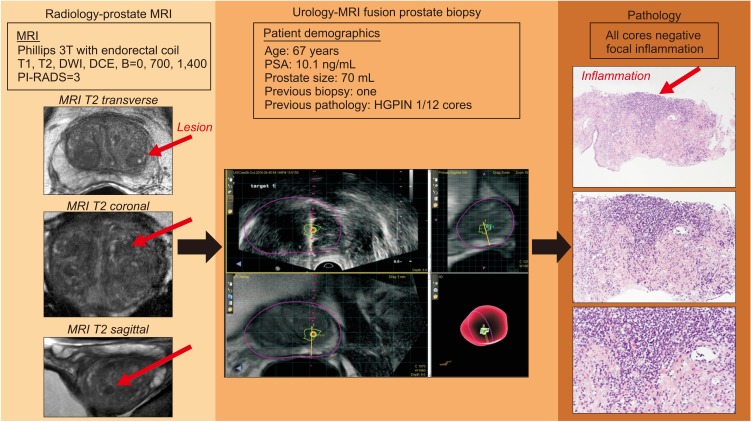
To investigate if inflammation as a potential cause of false-positive lesions from recent UroNav magnetic resonance imaging (MRI) fusion prostate biopsy patients.
MATERIALS AND METHODS
We retrospectively identified 43 men with 61 MRI lesions noted on prostate MRI before MRI ultrasound-guided fusion prostate biopsy. Men underwent MRI with 3T Siemens TIM Trio MRI system (Siemens AG, Germany), and lesions were identified and marked in DynaCAD system (Invivo Corporation, USA) with subsequent biopsy with MRI fusion with UroNav. We obtained targeted and standard 12-core needle biopsies. We retrospectively reviewed pathology reports for inflammation.
RESULTS
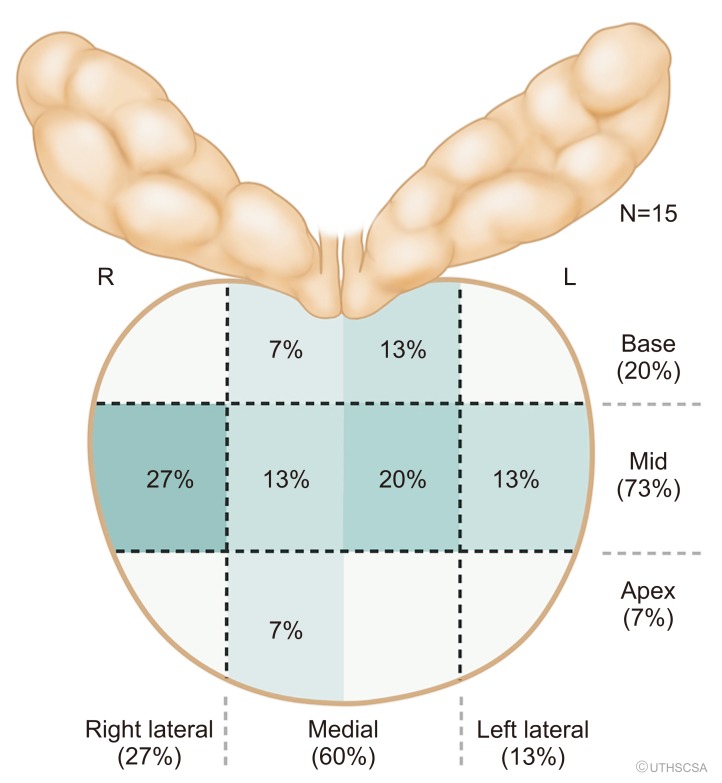
We noted a total of 43 (70.5%) false-positive lesions with 28 having no cancer on any cores, and 15 lesions with cancer noted on systematic biopsy but not in the target region. Of the men with cancer, 6 of the false positive lesions had inflammation in the location of the targeted region of interest (40.0%, 6/15). However, when we examine the 21/28 lesions with an identified lesion on MRI with no cancer in all cores, 54.5% had inflammation on prostate biopsy pathology (12/22, p=0.024). We noted the highest proportion of inflammation.
CONCLUSIONS
Inflammation can confound the interpretation of MRI by mimicking prostate cancer. We suggested focused efforts to differentiate inflammation and cancer on prostate MRI.
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. PMID: 28055103.
Article2. Haffty BG, Lawton CA, Sandler H. Watchful waiting-active surveillance in low-risk prostate cancer. JAMA Oncol. 2015; 1:688–689.
Article3. Filippou P, Welty CJ, Cowan JE, Perez N, Shinohara K, Carroll PR. Immediate versus delayed radical prostatectomy: updated outcomes following active surveillance of prostate cancer. Eur Urol. 2015; 68:458–463. PMID: 26138041.
Article4. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015; 313:390–397. PMID: 25626035.
Article5. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018; 378:1767–1777. PMID: 29552975.
Article6. Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016; 69:660–673. PMID: 26323946.7. Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Lisi D, Osimani M, et al. Magnetic resonance spectroscopic imaging (1H-MRSI) and dynamic contrast-enhanced magnetic resonance (DCE-MRI): pattern changes from inflammation to prostate cancer. Cancer Invest. 2010; 28:424–432. PMID: 20073578.
Article8. Cheng Y, Zhang X, Ji Q, Shen W. Xanthogranulomatous prostatitis: multiparametric MRI appearances. Clin Imaging. 2014; 38:755–757. PMID: 24852675.
Article9. Jyoti R, Jina NH, Haxhimolla HZ. In-gantry MRI guided prostate biopsy diagnosis of prostatitis and its relationship with PIRADS V.2 based score. J Med Imaging Radiat Oncol. 2017; 61:212–215. PMID: 27987276.
Article10. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging-reporting and data system: 2015, version 2. Eur Urol. 2016; 69:16–40. PMID: 26427566.11. Klotz L, Loblaw A, Sugar L, Moussa M, Berman DM, Van der Kwast T, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol. 2019; 75:300–309. PMID: 30017404.
Article12. Rosenkrantz AB, Verma S, Choyke P, Eberhardt SC, Eggener SE, Gaitonde K, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol. 2016; 196:1613–1618. PMID: 27320841.
Article13. Shukla-Dave A, Hricak H, Eberhardt SC, Olgac S, Muruganandham M, Scardino PT, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings--initial observations. Radiology. 2004; 231:717–724. PMID: 15163811.14. Nagel KN, Schouten MG, Hambrock T, Litjens GJ, Hoeks CM, ten Haken B, et al. Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology. 2013; 267:164–172. PMID: 23329653.
Article15. White NS, McDonald C, Farid N, Kuperman J, Karow D, Schenker-Ahmed NM, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014; 74:4638–4652. PMID: 25183788.
Article16. Liss MA, White NS, Parsons JK, Schenker-Ahmed NM, Rakow-Penner R, Kuperman JM, et al. MRI-derived restriction spectrum imaging cellularity index is associated with high grade prostate cancer on radical prostatectomy specimens. Front Oncol. 2015; 5:30. PMID: 25741473.
Article17. White NS, Leergaard TB, D'Arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum Brain Mapp. 2013; 34:327–346. PMID: 23169482.
Article18. White NS, McDonald CR, Farid N, Kuperman JM, Kesari S, Dale AM. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: quantitative comparison with high B-value DWI and ADC. AJNR Am J Neuroradiol. 2013; 34:958–964. S1PMID: 23139079.
Article19. Yamin G, Schenker-Ahmed NM, Shabaik A, Adams D, Bartsch H, Kuperman J, et al. Voxel level radiologic-pathologic validation of restriction spectrum imaging cellularity index with gleason grade in prostate cancer. Clin Cancer Res. 2016; 22:2668–2674. PMID: 27250935.
Article20. Othman AE, Falkner F, Martirosian P, Schraml C, Schwentner C, Nickel D, et al. Optimized fast dynamic contrast-enhanced magnetic resonance imaging of the prostate: effect of sampling duration on pharmacokinetic parameters. Invest Radiol. 2016; 51:106–112. PMID: 26447494.21. Mayer R, Simone CB 2nd, Skinner W, Turkbey B, Choykey P. Pilot study for supervised target detection applied to spatially registered multiparametric MRI in order to non-invasively score prostate cancer. Comput Biol Med. 2018; 94:65–73. PMID: 29407999.
Article22. Tekin A, Yuksel A, Tekin S, Gumrukcu G, Aslan AR, Sengor F. Post-prostatic massage examination for prediction of asymptomatic prostatitis in needle biopsies: a prospective study. J Urol. 2009; 182:564–568. discussion 568–9. PMID: 19524953.
Article23. De Luca S, Passera R, Fiori C, Bollito E, Cappia S, Mario Scarpa R, et al. Prostate health index and prostate cancer gene 3 score but not percent-free prostate specific antigen have a predictive role in differentiating histological prostatitis from PCa and other nonneoplastic lesions (BPH and HG-PIN) at repeat biopsy. Urol Oncol. 2015; 33:424.e17–424.e23.
Article24. Hendriks RJ, van der Leest MMG, Dijkstra S, Barentsz JO, Van Criekinge W, Hulsbergen-van de Kaa CA, et al. A urinary biomarker-based risk score correlates with multiparametric MRI for prostate cancer detection. Prostate. 2017; 77:1401–1407. PMID: 28853167.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prostate Imaging and Reporting and Data System Version 2.1: Limitations for Clinical Use
- Multiparametric MRI in the Detection of Clinically Significant Prostate Cancer
- A Cost-Benefit Comparison of Biparametric Magnetic Resonance Imaging Versus Conventional Prostate Cancer Screening
- Magnetic resonance imaging-transrectal ultrasound fusion image-guided prostate biopsy: Current status of the cancer detection and the prospects of tailor-made medicine of the prostate cancer
- The Clinical Value of Performing an MRI before Prostate Biopsy