Clin Endosc.
2019 Jul;52(4):334-339. 10.5946/ce.2019.004.
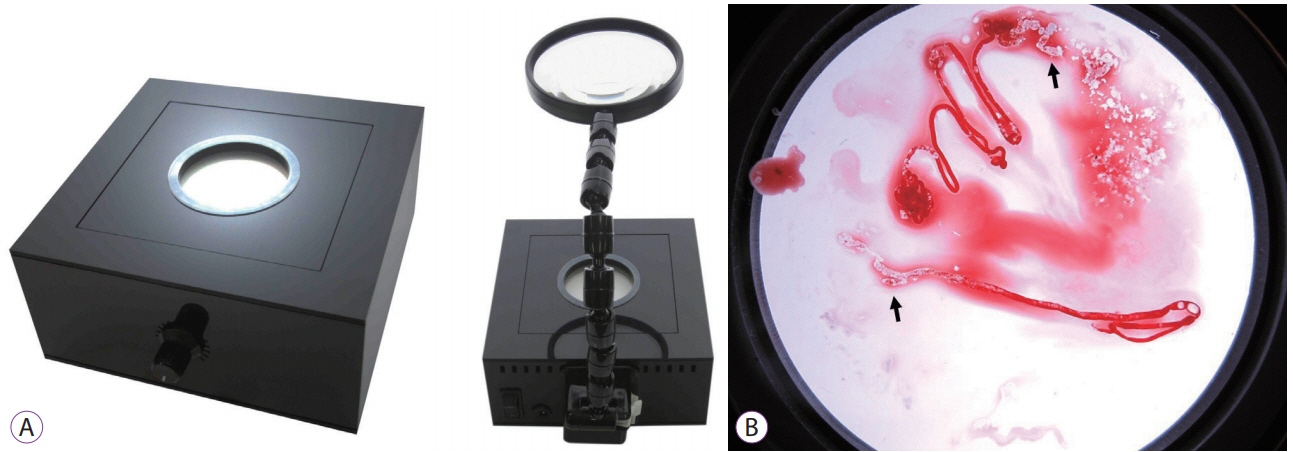
A “Back Light System†for Identification of Sites for Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Solid Pancreatic Masses: A Prospective, Randomized Study with a Crossover Design
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama, Japan. rharada927@yahoo.co.jp
- 2Department of Pathology, Okayama University Hospital, Okayama, Japan.
- KMID: 2455629
- DOI: http://doi.org/10.5946/ce.2019.004
Abstract
- BACKGROUND/AIMS
We applied a back light system (BLS) with a magnifying glass to improve the ability to assess the adequacy of specimen sampling using endosonography. We conducted this study to evaluate the efficacy of the BLS in sampling of specimens by endoscopic ultrasound-guided fine needle aspiration of solid pancreatic masses.
METHODS
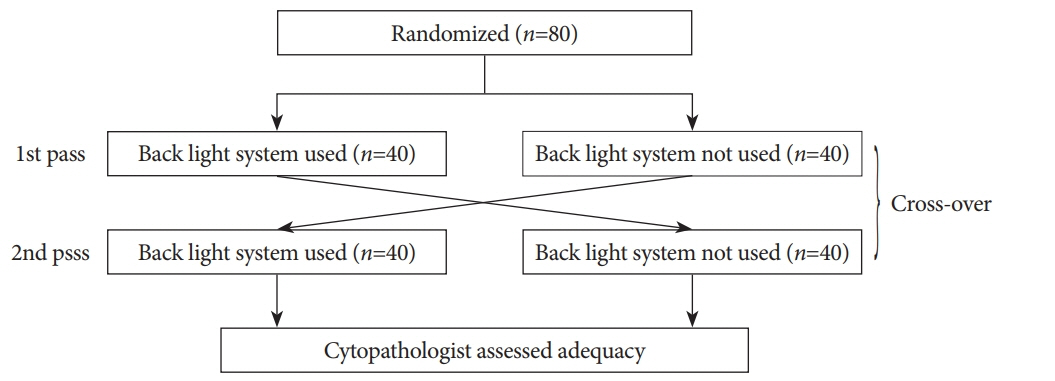
This was a prospective, randomized, crossover, single-center clinical trial. An endosonographer evaluated adequacy on gross visual inspection and identified whitish specimen sampling sites with and without the BLS according to a randomization sequence in the first and second passes with a 25-G needle. On cytological evaluation, the presence of well-defined pancreatic ductal epithelium was evaluated by a cytopathologist who was blinded to any clinical information.
RESULTS
A total of 80 consecutive patients were eligible during the study period. Adequacy was observed for 52 specimens (65%) with the BLS and 54 (68%) without the BLS (p=0.88). In assessment of specimen adequacy on gross examination, only fair agreement was observed both with and without BLS (kappa score 0.40 and 0.29, respectively).
CONCLUSIONS
The BLS did not influence the ability to identify specimen sampling sites or reliable assessment of specimen site adequacy using gross visual inspection.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Unfortunately, a “Back Light System” As a Global Positioning System Failed to Guide the Route in 25-G Fine-Needle Aspiration
Rungsun Rerknimitr, Phonthep Angsuwatcharakon
Clin Endosc. 2019;52(4):295-296. doi: 10.5946/ce.2019.104.
Reference
-
1. Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992; 38:172–173.
Article2. Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000; 51:184–190.
Article3. Kim J, Ryu JK, Park JM, et al. Clinical factors associated with accuracy of EUS-FNA for pancreatic or peripancreatic solid mass without on-site cytopathologists. J Gastroenterol Hepatol. 2014; 29:887–892.
Article4. Ishiwatari H, Hayashi T, Kawakami H, et al. Randomized trial comparing a side-port needle and standard needle for EUS-guided histology of pancreatic lesions. Gastrointest Endosc. 2016; 84:670–678.
Article5. Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009; 70:1093–1097.
Article6. Carrara S, Anderloni A, Jovani M, et al. A prospective randomized study comparing 25-G and 22-G needles of a new platform for endoscopic ultrasound-guided fine needle aspiration of solid masses. Dig Liver Dis. 2016; 48:49–54.
Article7. Mohamadnejad M, Mullady D, Early DS, et al. Increasing number of passes beyond 4 does not increase sensitivity of detection of pancreatic malignancy by endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2017; 15:1071–1078. e2.
Article8. Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015; 81:1401–1407.9. Chen JY, Ding QY, Lv Y, et al. Slow-pull and different conventional suction techniques in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid lesions using 22-gauge needles. World J Gastroenterol. 2016; 22:8790–8797.
Article10. Hashimoto S, Taguchi H, Higashi M, et al. Diagnostic efficacy of liquid-based cytology for solid pancreatic lesion samples obtained with endoscopic ultrasound-guided fine-needle aspiration: propensity scorematched analysis. Dig Endosc. 2017; 29:608–616.
Article11. Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003; 98:1289–1294.
Article12. Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011; 106:1705–1710.
Article13. Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013; 48:973–981.
Article14. Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014; 29:697–705.
Article15. Keswani RN, Krishnan K, Wani S, Keefer L, Komanduri S. Addition of endoscopic ultrasound (EUS)-guided fine needle aspiration and on-site cytology to EUS-guided fine needle biopsy increases procedure time but not diagnostic accuracy. Clin Endosc. 2014; 47:242–247.
Article16. Hayashi T, Ishiwatari H, Yoshida M, et al. Rapid on-site evaluation by endosonographer during endoscopic ultrasound-guided fine needle aspiration for pancreatic solid masses. J Gastroenterol Hepatol. 2013; 28:656–663.
Article17. Minami D, Takigawa N, Inoue H, Hotta K, Tanimoto M, Kiura K. Rapid on-site evaluation with BIOEVALUATOR® during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing pulmonary and mediastinal diseases. Ann Thorac Med. 2014; 9:14–17.18. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
Article19. Wani S, Wallace MB, Cohen J, et al. Quality indicators for EUS. Gastrointest Endosc. 2015; 81:67–80.
Article20. Katanuma A, Maguchi H, Yane K, et al. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci. 2013; 58:2093–2099.
Article21. Minaga K, Kitano M, Yamashita Y. Surgically resected needle tract seeding following endoscopic ultrasound-guided fine-needle aspiration in pancreatic cancer. J Hepatobiliary Pancreat Sci. 2015; 22:708–709.
Article22. Tomonari A, Katanuma A, Matsumori T, et al. Resected tumor seeding in stomach wall due to endoscopic ultrasonography-guided fine needle aspiration of pancreatic adenocarcinoma. World J Gastroenterol. 2015; 21:8458–8461.
Article23. Harada R, Kato H, Fushimi S, et al. An expanded training program for endosonographers improved self-diagnosed accuracy of endoscopic ultrasound-guided fine-needle aspiration cytology of the pancreas. Scand J Gastroenterol. 2014; 49:1119–1123.
Article24. Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study). Gastrointest Endosc. 2015; 81:177–185.
Article25. Nguyen YP, Maple JT, Zhang Q, et al. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2009; 69:1264–1270.
Article26. Matsumoto K, Ueki M, Takeda Y, et al. Development of a device for detecting target specimens from EUS-guided FNA samples. Endosc Int Open. 2015; 3:E662–E664.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- How to optimize the diagnostic yield of endoscopic ultrasoundguided fine-needle sampling in solid pancreatic lesions from a technical perspective
- Endoscopic Ultrasound-Guided Direct Intervention for Solid Pancreatic Tumors
- Role of Repeated Endoscopic Ultrasound-Guided Fine Needle Aspiration for Inconclusive Initial Cytology Result
- Endoscopic Ultrasound-Guided Fine Needle Aspiration in Cystic Pancreatic Lesions
- How Can We Get the Best Results with Endoscopic Ultrasound-Guided Fine Needle Aspiration?