Clin Endosc.
2019 Jul;52(4):301-305. 10.5946/ce.2019.024.
Current Status of Endoscopic Ultrasonography in Gastrointestinal Subepithelial Tumors
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea.
- 3Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, CA, USA. jooha@stanford.edu
- KMID: 2455624
- DOI: http://doi.org/10.5946/ce.2019.024
Abstract
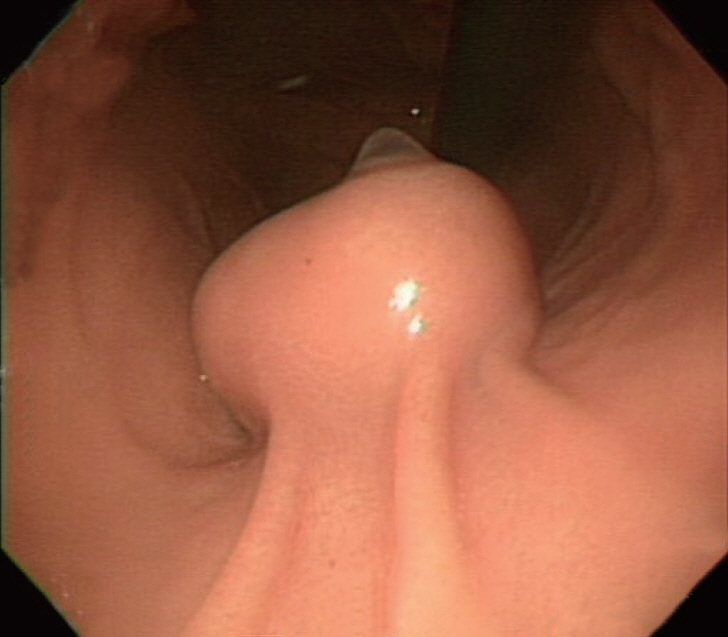
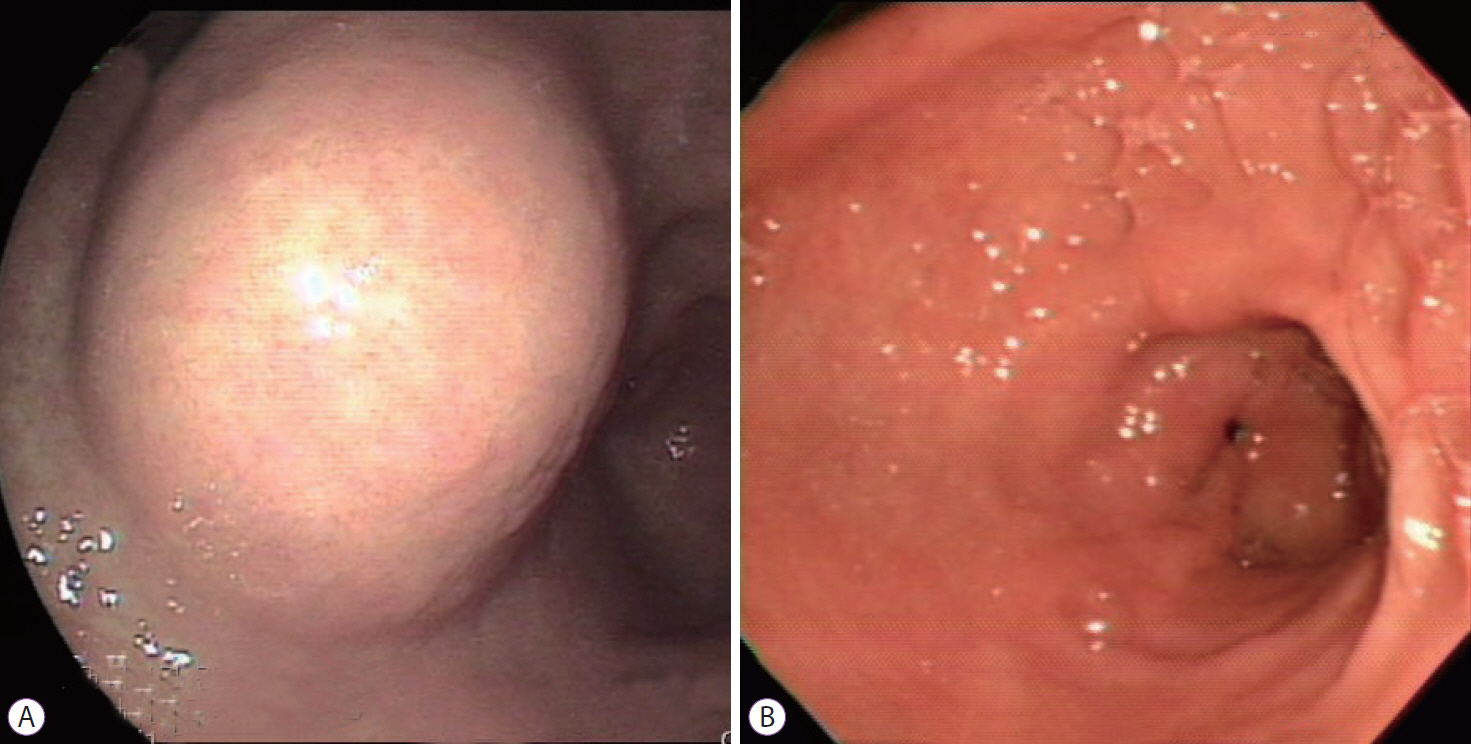
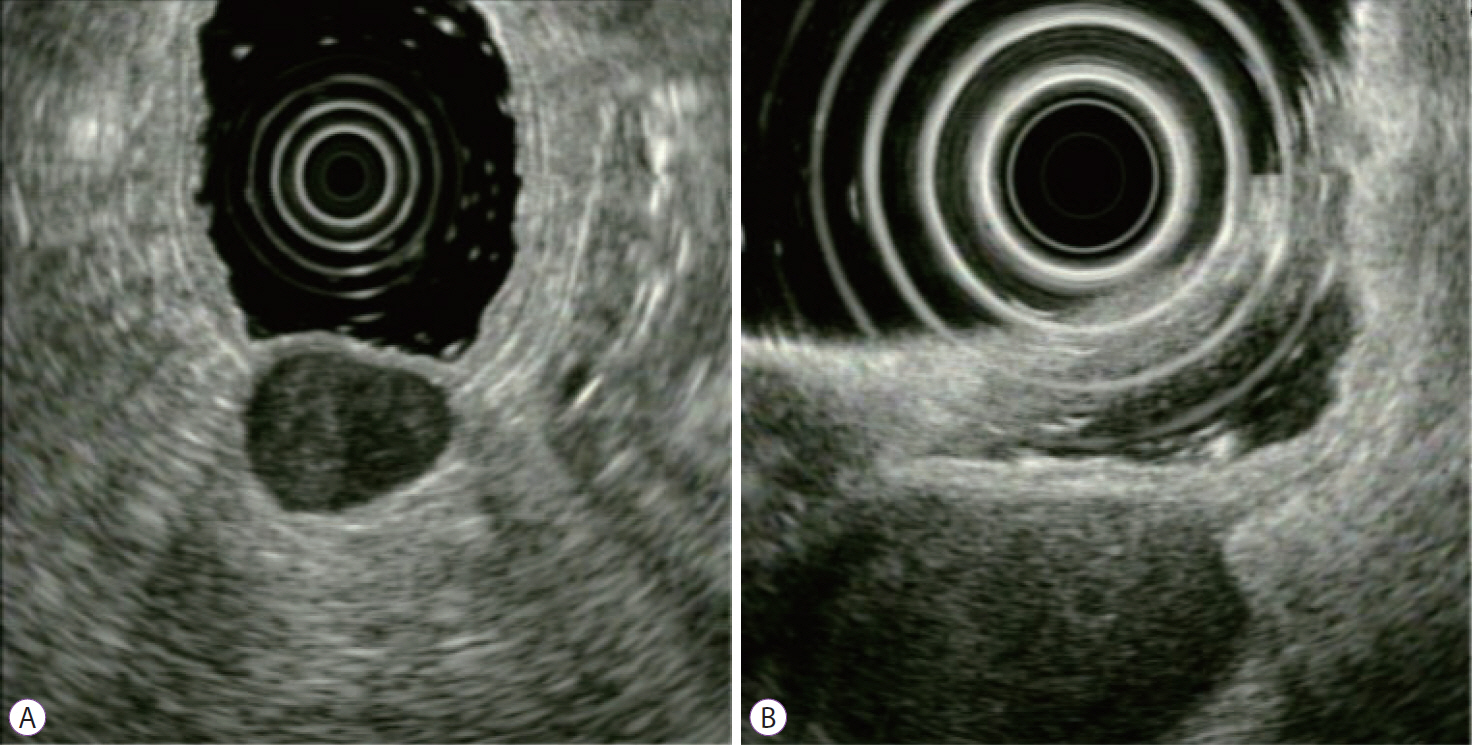
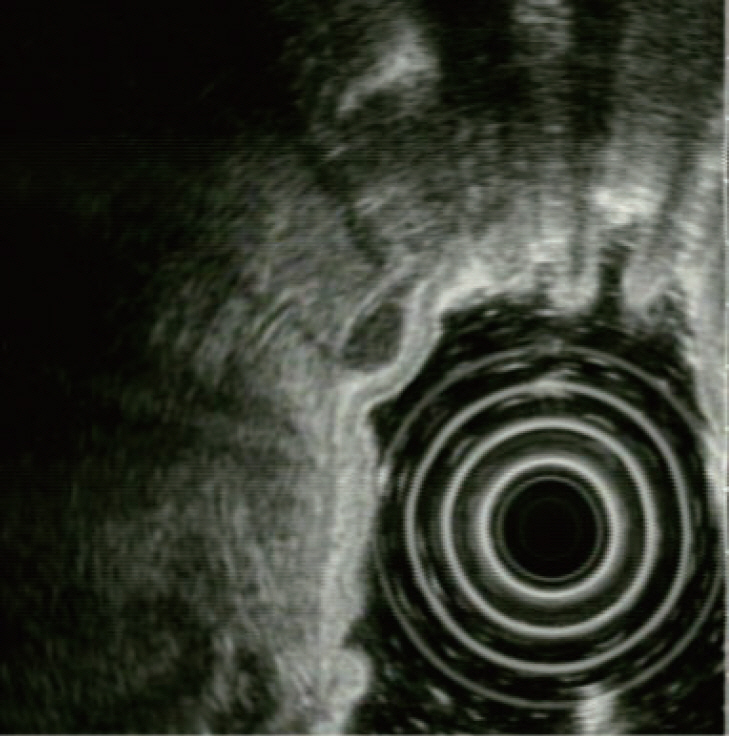
- Gastrointestinal subepithelial tumors (GSTs) are usually detected incidentally on endoscopic or radiologic examinations. In conventional endoscopy, a GST usually presents as a protuberant lesion with an intact mucosal surface. As the lesion is located beneath the mucosal layer of the gastrointestinal tract, conventional biopsy typically does not reveal the pathologic diagnosis. First, a GST should be differentiated from an extrinsic compression through the positional change of the patient during conventional endoscopic examination. In cases of GSTs originating from the gastrointestinal wall, endoscopic ultrasonography (EUS) can be beneficial for narrowing the differential diagnosis through delineation of echo findings and by determining the layer of origin. EUS findings can also help determine the management strategies for GSTs by making a differential diagnosis according to malignant potential.
Keyword
MeSH Terms
Figure
Reference
-
1. Song JH, Kim SG, Chung SJ, Kang HY, Yang SY, Kim YS. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy. 2015; 47:675–679.
Article2. Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991; 5:20–23.3. Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010; 16:439–444.
Article4. Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013; 25:479–489.
Article5. Gong EJ, Kim DH. Endoscopic ultrasonography in the diagnosis of gastric subepithelial lesions. Clin Endosc. 2016; 49:425–433.
Article6. Moon JS. Role of endoscopic ultrasonography in guiding treatment plans for upper gastrointestinal subepithelial tumors. Clin Endosc. 2016; 49:220–225.
Article7. Kim YW, Kim GH, Kim DU, et al. The clinical significance of extraluminal compressions according to the site of the stomach. Korean J Gastrointest Endosc. 2009; 39:125–130.8. Pyeon SI, Kim GH, Yoon JB, Jeon HK, Lee BE. Clinical significance of extraluminal compressions according to the site of the esophagus. Korean J Helicobacter Up Gastrointest Res. 2017; 17:127–131.
Article9. Kim TO. Colorectal subepithelial lesions. Clin Endosc. 2015; 48:302–307.
Article10. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006; 30:477–489.11. Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004; 126:301–307.
Article12. Brand B, Oesterhelweg L, Binmoeller KF, et al. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis. 2002; 34:290–297.
Article13. Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000; 46:88–92.
Article14. Kim MN, Kang SJ, Kim SG, et al. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography. Gut Liver. 2013; 7:642–647.
Article15. Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006; 130:2217–2228.
Article16. Menon L, Buscaglia JM. Endoscopic approach to subepithelial lesions. Therap Adv Gastroenterol. 2014; 7:123–130.
Article17. Kim GH, Cho YK, Kim EY, et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014; 49:347–354.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidental Gastrointestinal Subepithelial Mass
- Endoscopic Ultrasonography in the Diagnosis of Gastric Subepithelial Lesions
- Contrast Enhanced Endoscopic Ultrasound Imaging for Gastrointestinal Subepithelial Tumors
- Current Techniques for Treating Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
- Endoscopic Treatment of Subepithelial Tumors