Clin Endosc.
2019 Jul;52(4):306-313. 10.5946/ce.2019.056.
Contrast Enhanced Endoscopic Ultrasound Imaging for Gastrointestinal Subepithelial Tumors
- Affiliations
-
- 1Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan. kitano@wakayama-med.ac.jp
- KMID: 2455625
- DOI: http://doi.org/10.5946/ce.2019.056
Abstract
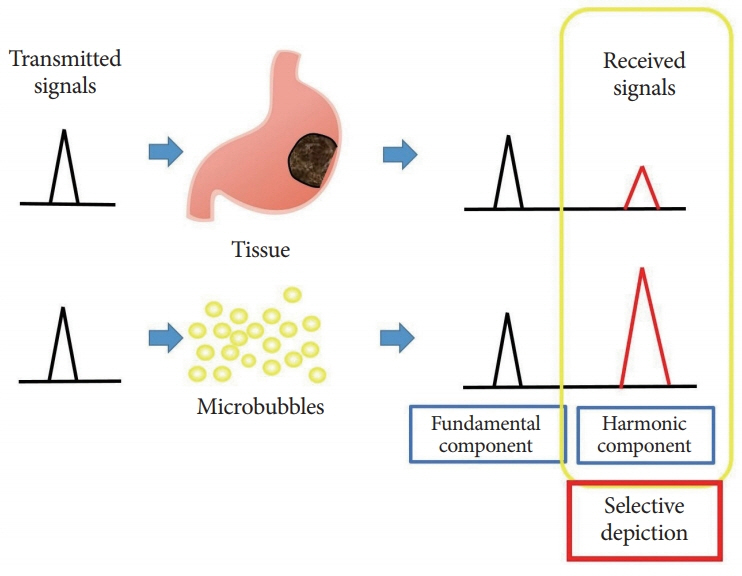
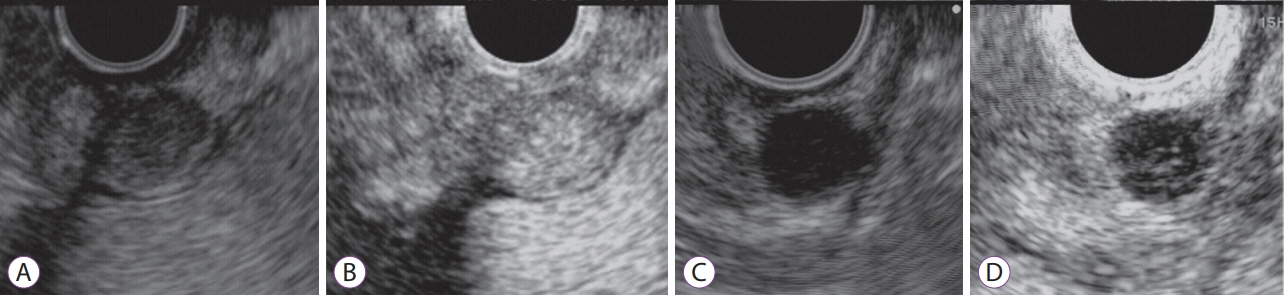
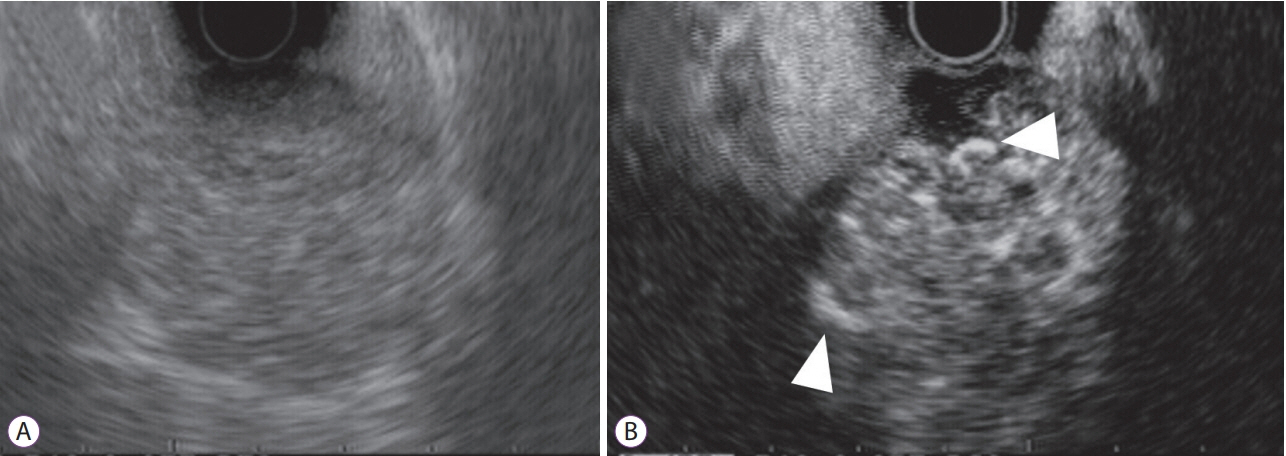
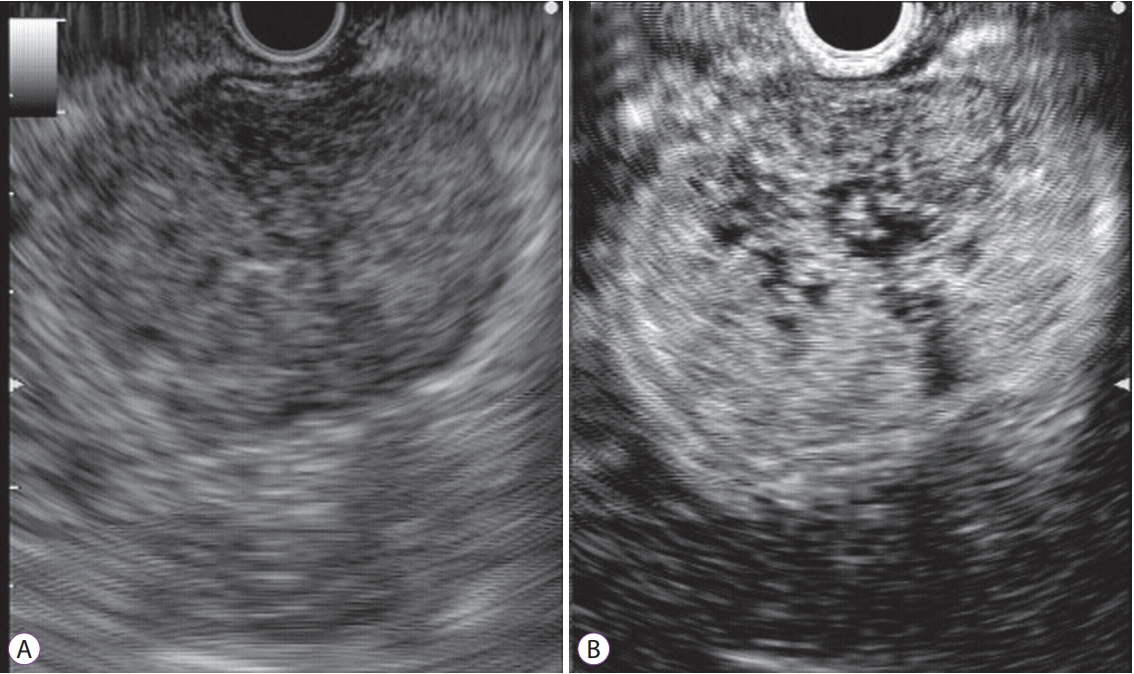
- Subepithelial tumors are divided into benign subepithelial and potentially malignant gastrointestinal stromal tumors. It is difficult to distinguish between these tumor types. Contrast-enhanced harmonic endoscopic ultrasound is reportedly useful for diagnosing subepithelial tumors, can be safely and easily performed by understanding the principle and method, and can be used to distinguish between tumor types with high sensitivity on the basis of differences in contrast effect. The generated image shows a hyper-enhancement pattern in gastrointestinal stromal tumors (sensitivity, 78%-100%; specificity, 60%-100%; accuracy, 60%-100%) and hypo-enhancement pattern in benign subepithelial tumors. Contrast-enhanced harmonic endoscopic ultrasound can be used to estimate the malignancy potential of gastrointestinal stromal tumors by evaluating the uniformity of the contrast and the blood vessels inside the tumor, with abnormal intra-tumor blood vessels, heterogeneous enhancement, and non-enhancing spots suggesting malignancy. Contrast-enhanced harmonic endoscopic ultrasound has a higher sensitivity than other imaging modalities for the detection of vascularity within gastrointestinal stromal tumors. Additionally, it has been reported that treatment effects can be estimated by evaluating the blood flow in the gastrointestinal stromal tumor before and after treatment with tyrosine kinase inhibitors using contrast-enhanced ultrasound. However, there will be subjective-bias and the results depends on the performer's skill.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Efficacy and Safety of Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
Cicilia Marcella, Shakeel Sarwar, Hui Ye, Rui Hua Shi
Clin Endosc. 2020;53(4):458-465. doi: 10.5946/ce.2019.121.
Reference
-
1. Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006; 130:2217–2228.
Article2. Min YW, Park HN, Min BH, Choi D, Kim KM, Kim S. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg. 2015; 19:631–638.
Article3. Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000; 15:1293–1301.4. Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996; 33:817–872.
Article5. Okai T, Minamoto T, Ohtsubo K, et al. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom Imaging. 2003; 28:301–307.
Article6. Chak A, Canto MI, Rösch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997; 45:468–473.
Article7. Seo SW, Hong SJ, Han JP, et al. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013; 14:647–653.
Article8. Kannengiesser K, Mahlke R, Petersen F, et al. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012; 47:1515–1520.
Article9. Kamata K, Takenaka M, Kitano M, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of submucosal tumors of the upper gastrointestinal tract. J Gastroenterol Hepatol. 2017; 32:1686–1692.
Article10. Pesenti C, Bories E, Caillol F, et al. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: a retrospective study. Endosc Ultrasound. 2019; 8:43–49.
Article11. Lee HS, Cho CM, Kwon YH, Nam SY. Predicting malignancy risk in gastrointestinal subepithelial tumors with contrast-enhanced harmonic endoscopic ultrasonography using perfusion analysis software. Gut Liver. 2019; 13:161–168.
Article12. Ignee A, Jenssen C, Hocke M, et al. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc Ultrasound. 2017; 6:55–60.
Article13. Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound. 2015; 43:89–97.
Article14. Park HY, Jeon SW, Lee HS, et al. Can contrast-enhanced harmonic endosonography predict malignancy risk in gastrointestinal subepithelial tumors? Endosc Ultrasound. 2016; 5:384–389.
Article15. Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2011; 73:227–237.
Article16. Lassau N, Chami L, Koscielny S, et al. Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinib. Invest New Drugs. 2012; 30:765–771.
Article17. Chhoda A, Jain D, Surabhi VR, Singhal S. Contrast enhanced harmonic endoscopic ultrasound: a novel approach for diagnosis and management of gastrointestinal stromal tumors. Clin Endosc. 2018; 51:215–221.
Article18. Desser TS, Jeffrey RB. Tissue harmonic imaging techniques: physical principles and clinical applications. Semin Ultrasound CT MR. 2001; 22:1–10.
Article19. Kollmann C. New sonographic techniques for harmonic imaging--underlying physical principles. Eur J Radiol. 2007; 64:164–172.
Article20. Sanchez MV, Varadarajulu S, Napoleon B. EUS contrast agents: what is available, how do they work, and are they effective? Gastrointest Endosc. 2009; 69(2 Suppl):S71–S77.21. Kitano M, Kudo M, Sakamoto H, et al. Preliminary study of contrast-enhanced harmonic endosonography with second-generation contrast agents. J Med Ultrason (2001). 2008; 35:11–18.
Article22. Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008; 67:141–150.
Article23. Săftoiu A, Dietrich CF, Vilmann P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy. 2012; 44:612–617.
Article24. Fukuta N, Kitano M, Maekawa K, Chikugo T, Kudo M. Estimation of the malignant potential of gastrointestinal stromal tumors: the value of contrast-enhanced coded phase-inversion harmonics US. J Gastroenterol. 2005; 40:247–255.
Article25. Chen WT, Huang CJ, Wu MT, Yang SF, Su YC, Chai CY. Hypoxia-inducible factor-1alpha is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol. 2005; 35:207–213.26. Takahashi R, Tanaka S, Hiyama T, et al. Hypoxia-inducible factor-1alpha expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003; 10:797–802.27. Takahashi R, Tanaka S, Kitadai Y, et al. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003; 64:266–274.
Article28. Van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs). Eur J Cancer. 2002; 38 Suppl 5:S60–S65.
Article29. Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007; 25:1753–1759.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Common Gastric Subepithelial Tumors in Koreans
- Incidental Gastrointestinal Subepithelial Mass
- The Diagnosis of Subepithelial Lesions in the Upper Gastrointestinal Tract
- Modern ultrasound imaging of pancreatic tumors
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors