Yonsei Med J.
2012 Sep;53(5):875-885.
Hepatitis B Precore Protein: Pathogenic Potential and Therapeutic Promise
- Affiliations
-
- 1Research & Molecular Development, Victorian Infectious Diseases Reference Laboratory, Melbourne, Victoria, Australia. renae.walsh@mh.org.au
Abstract
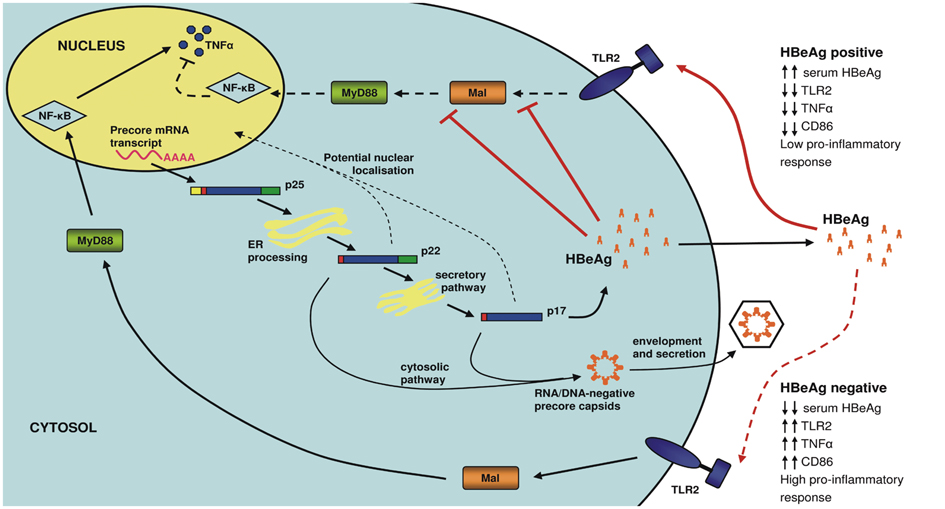
- Hepatitis B virus (HBV), a small and economically packaged double-stranded DNA virus, represents an enormous global health care burden. In spite of an effective vaccine, HBV is endemic in many countries. Chronic hepatitis B (CHB) results in the development of significant clinical outcomes such as liver disease and hepatocellular carcinoma (HCC), which are associated with high mortality rates. HBV is a non-cytopathic virus, with the host's immune response responsible for the associated liver damage. Indeed, HBV appears to be a master of manipulating and modulating the immune response to achieve persistent and chronic infection. The HBV precore protein or hepatitis B e antigen (HBeAg) is a key viral protein involved in these processes, for instance though the down-regulation of the innate immune response. The development of new therapies that target viral proteins, such as HBeAg, which regulates of the immune system, may offer a new wave of potential therapeutics to circumvent progression to CHB and liver disease.
MeSH Terms
Figure
Reference
-
1. Kane MA. Global status of hepatitis B immunisation. Lancet. 1996. 348:696.
Article2. Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004. 11:97–107.
Article3. Kim BK, Revill PA, Ahn SH. HBV genotypes: relevance to natural history, pathogenesis and treatment of chronic hepatitis B. Antivir Ther. 2011. 16:1169–1186.
Article4. Lai CL, Yuen MF. The natural history of chronic hepatitis B. J Viral Hepat. 2007. 14:Suppl 1. 6–10.
Article5. McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int. 2009. 3:334–342.
Article6. Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003. 38:1075–1086.
Article7. Chang C, Enders G, Sprengel R, Peters N, Varmus HE, Ganem D. Expression of the precore region of an avian hepatitis B virus is not required for viral replication. J Virol. 1987. 61:3322–3325.
Article8. Chen MT, Billaud JN, Sällberg M, Guidotti LG, Chisari FV, Jones J, et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci U S A. 2004. 101:14913–14918.
Article9. Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990. 87:6599–6603.
Article10. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007. 45:507–539.
Article11. Ou JH. Molecular biology of hepatitis B virus e antigen. J Gastroenterol Hepatol. 1997. 12:S178–S187.
Article12. Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: recent advances. J Gastroenterol Hepatol. 2011. 26:Suppl 1. 123–130.
Article13. Kao JH. Molecular epidemiology of hepatitis B virus. Korean J Intern Med. 2011. 26:255–261.
Article14. Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002. 347:168–174.
Article15. Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007. 45:102–110.
Article16. Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012. 61:Suppl 1. i6–17.
Article17. McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009. 49:5 Suppl. S45–S55.
Article18. Herkel J, Carambia A. Let it B in viral hepatitis? J Hepatol. 2011. 55:5–7.
Article19. Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006. 27:352–357.
Article20. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999. 18:6853–6866.
Article21. Thompson AJ, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol. 2007. 85:435–445.
Article22. Roy CR, Mocarski ES. Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol. 2007. 8:1179–1187.
Article23. Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008. 128:400–408.
Article24. Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011. 55:762–769.
Article25. Riordan SM, Skinner N, Kurtovic J, Locarnini S, Visvanathan K. Reduced expression of toll-like receptor 2 on peripheral monocytes in patients with chronic hepatitis B. Clin Vaccine Immunol. 2006. 13:972–974.
Article26. Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009. 49:1132–1140.
Article27. Revill P, Yuen L, Walsh R, Perrault M, Locarnini S, Kramvis A. Bioinformatic analysis of the hepadnavirus e-antigen and its precursor identifies remarkable sequence conservation in all orthohepadnaviruses. J Med Virol. 2010. 82:104–115.
Article28. Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008. 453:667–671.
Article29. Han SH, Martin P, Edelstein M, Hu R, Kunder G, Holt C, et al. Conversion from intravenous to intramuscular hepatitis B immune globulin in combination with lamivudine is safe and cost-effective in patients receiving long-term prophylaxis to prevent hepatitis B recurrence after liver transplantation. Liver Transpl. 2003. 9:182–187.
Article30. Neumann AU, Phillips S, Levine I, Ijaz S, Dahari H, Eren R, et al. Novel mechanism of antibodies to hepatitis B virus in blocking viral particle release from cells. Hepatology. 2010. 52:875–885.
Article31. Schilling R, Ijaz S, Davidoff M, Lee JY, Locarnini S, Williams R, et al. Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions. J Virol. 2003. 77:8882–8892.
Article32. Maruyama T, McLachlan A, Iino S, Koike K, Kurokawa K, Milich DR. The serology of chronic hepatitis B infection revisited. J Clin Invest. 1993. 91:2586–2595.
Article33. Chen M, Sällberg M, Hughes J, Jones J, Guidotti LG, Chisari FV, et al. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol. 2005. 79:3016–3027.
Article34. Milich DR, Chen MK, Hughes JL, Jones JE. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J Immunol. 1998. 160:2013–2021.35. Walsh R, Nuttall S, Revill P, Colledge D, Cabuang L, Soppe S, et al. Targeting the hepatitis B virus precore antigen with a novel IgNAR single variable domain intrabody. Virology. 2011. 411:132–141.
Article36. Dienes HP, Gerken G, Goergen B, Heermann K, Gerlich W, Meyer zum Büschenfelde KH. Analysis of the precore DNA sequence and detection of precore antigen in liver specimens from patients with anti-hepatitis B e-positive chronic hepatitis. Hepatology. 1995. 21:1–7.
Article37. Kimura T, Ohno N, Terada N, Rokuhara A, Matsumoto A, Yagi S, et al. Hepatitis B virus DNA-negative dane particles lack core protein but contain a 22-kDa precore protein without C-terminal arginine-rich domain. J Biol Chem. 2005. 280:21713–21719.
Article38. Messageot F, Salhi S, Eon P, Rossignol JM. Proteolytic processing of the hepatitis B virus e antigen precursor. Cleavage at two furin consensus sequences. J Biol Chem. 2003. 278:891–895.39. Takahashi K, Machida A, Funatsu G, Nomura M, Usuda S, Aoyagi S, et al. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983. 130:2903–2907.40. Takahashi K, Brotman B, Usuda S, Mishiro S, Prince AM. Full-genome sequence analyses of hepatitis B virus (HBV) strains recovered from chimpanzees infected in the wild: implications for an origin of HBV. Virology. 2000. 267:58–64.
Article41. Watts NR, Conway JF, Cheng N, Stahl SJ, Steven AC, Wingfield PT. Role of the propeptide in controlling conformation and assembly state of hepatitis B virus e-antigen. J Mol Biol. 2011. 409:202–213.
Article42. Steven AC, Conway JF, Cheng N, Watts NR, Belnap DM, Harris A, et al. Structure, assembly, and antigenicity of hepatitis B virus capsid proteins. Adv Virus Res. 2005. 64:125–164.
Article43. Chen RY, Edwards R, Shaw T, Colledge D, Delaney WE 4th, Isom H, et al. Effect of the G1896A precore mutation on drug sensitivity and replication yield of lamivudine-resistant HBV in vitro. Hepatology. 2003. 37:27–35.
Article44. Lamberts C, Nassal M, Velhagen I, Zentgraf H, Schröder CH. Precore-mediated inhibition of hepatitis B virus progeny DNA synthesis. J Virol. 1993. 67:3756–3762.
Article45. Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989. 2:588–591.
Article46. Buckwold VE, Xu Z, Chen M, Yen TS, Ou JH. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J Virol. 1996. 70:5845–5851.
Article47. Scaglioni PP, Melegari M, Wands JR. Posttranscriptional regulation of hepatitis B virus replication by the precore protein. J Virol. 1997. 71:345–353.
Article48. Locarnini S, Shaw T, Dean J, Colledge D, Thompson A, Li K, et al. Cellular response to conditional expression of the hepatitis B virus precore and core proteins in cultured hepatoma (Huh-7) cells. J Clin Virol. 2005. 32:113–121.
Article49. Locarnini S, Mason WS. Cellular and virological mechanisms of HBV drug resistance. J Hepatol. 2006. 44:422–431.
Article50. Warner N, Locarnini S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology. 2008. 48:88–98.
Article51. Yuen L, Ayers A, Littlejohn M, Edwards R, Locarnini S. Evolution of the hepatitis B surface antigen in European and Asian HBV genotypes during the era of antiviral therapy. Hepatol Int. 2011. 5:3–558. Abstract PP05-159.52. Lever A, Waters J, Brook G, Karayiannis P, Thomas H, editors. Zuckerman AJ, editor. Treatment of chronic hepatitis B virus infection with monoclonal antibody to the hepatitis B virus surface antigen in two patients with hypogammaglobulinaemia. Viral Hep & Liver Dis. 1987. New York: Alan R Liss Inc;961–962.53. Lever AM, Waters J, Brook MG, Karayiannis P, Thomas HC. Monoclonal antibody to HBsAg for chronic hepatitis B virus infection with hypogammaglobulinaemia. Lancet. 1990. 335:1529.
Article54. Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008. 47:428–434.
Article55. National Institutes of Health. RFA-DK-07-011. Hepatitis B clinical research network. Released: December 2007. http://grants.nih.gov/grants/guide/rfa-files/RFA-DK-07-011.html.56. Nuttall SD, Krishnan UV, Doughty L, Pearson K, Ryan MT, Hoogenraad NJ, et al. Isolation and characterization of an IgNAR variable domain specific for the human mitochondrial translocase receptor Tom70. Eur J Biochem. 2003. 270:3543–3554.
Article57. Roux KH, Greenberg AS, Greene L, Strelets L, Avila D, McKinney EC, et al. Structural analysis of the nurse shark (new) antigen receptor (NAR): molecular convergence of NAR and unusual mammalian immunoglobulins. Proc Natl Acad Sci U S A. 1998. 95:11804–11809.
Article58. Ewert S, Cambillau C, Conrath K, Plückthun A. Biophysical properties of camelid V(HH) domains compared to those of human V(H)3 domains. Biochemistry. 2002. 41:3628–3636.
Article59. Nuttall SD, Humberstone KS, Krishnan UV, Carmichael JA, Doughty L, Hattarki M, et al. Selection and affinity maturation of IgNAR variable domains targeting Plasmodium falciparum AMA1. Proteins. 2004. 55:187–197.
Article60. Stanfield RL, Dooley H, Flajnik MF, Wilson IA. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science. 2004. 305:1770–1773.
Article61. Streltsov VA, Varghese JN, Carmichael JA, Irving RA, Hudson PJ, Nuttall SD. Structural evidence for evolution of shark Ig new antigen receptor variable domain antibodies from a cell-surface receptor. Proc Natl Acad Sci U S A. 2004. 101:12444–12449.
Article62. Streltsov VA, Carmichael JA, Nuttall SD. Structure of a shark IgNAR antibody variable domain and modeling of an early-developmental isotype. Protein Sci. 2005. 14:2901–2909.
Article63. Dandri M, Volz TK, Lütgehetmann M, Petersen J. Animal models for the study of HBV replication and its variants. J Clin Virol. 2005. 34:Suppl 1. S54–S62.
Article64. Ladner SK, Otto MJ, Barker CS, Zaifert K, Wang GH, Guo JT, et al. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother. 1997. 41:1715–1720.
Article65. Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987. 84:1005–1009.
Article66. Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005. 5:215–229.
Article67. Eren R, Lubin I, Terkieltaub D, Ben-Moshe O, Zauberman A, Uhlmann R, et al. Human monoclonal antibodies specific to hepatitis B virus generated in a human/mouse radiation chimera: the Trimera system. Immunology. 1998. 93:154–161.
Article68. Eren R, Ilan E, Nussbaum O, Lubin I, Terkieltaub D, Arazi Y, et al. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology. 2000. 32:588–596.
Article69. Ilan E, Burakova T, Dagan S, Nussbaum O, Lubin I, Eren R, et al. The hepatitis B virus-trimera mouse: a model for human HBV infection and evaluation of anti-HBV therapeutic agents. Hepatology. 1999. 29:553–562.
Article70. Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001. 33:981–988.
Article71. Walter E, Keist R, Niederöst B, Pult I, Blum HE. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology. 1996. 24:1–5.
Article72. Menne S, Cote PJ. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol. 2007. 13:104–124.
Article73. Hamatake RK, Lau JYN. Hepatitis B and D Protocols, vol 2. Methods in Molecular Medicine. 2004. vol 96:Humana Press.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on the Emergence of Precore Mutant in HBV Infection
- Mutations in Hepatitis B Virus Precore and Core Promotor in Children with Chronic Hepatitis B Infection - Comparison Between Vertical and Non-vertical Transmission
- Interferon Treatment on HBeAg Positive Chronic Hepatitis B with HBV Precore Mutant in Liver Tissue
- Mutations in Hepatitis B Virus Precore, Core Promoter, and "a" Determinant in Children with Chronic Hepatitis B Virus Infection
- Naturally Occurring Mutations of Hepatitis B virus and Hepatitis C Virus in Korean Chronic Patients by Distinct CD4 T Cell Responses