Anat Cell Biol.
2017 Jun;50(2):77-85. 10.5115/acb.2017.50.2.77.
The molecular mechanism for nuclear transport and its application
- Affiliations
-
- 1Department of Anatomy, Pusan National University School of Medicine, Yangsan, Korea. hedgehog@pusan.ac.kr
- 2BEER, Busan Society of Evidence-Based mEdicine and Research, Busan, Korea.
- 3Gene and Cell Therapy Research Center for Vessel-associated Diseases, Pusan National University, Yangsan, Korea.
- KMID: 2451231
- DOI: http://doi.org/10.5115/acb.2017.50.2.77
Abstract
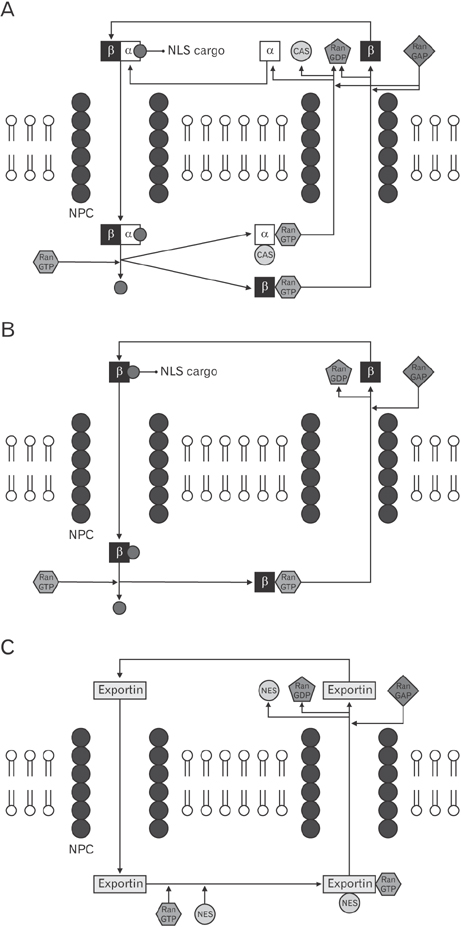
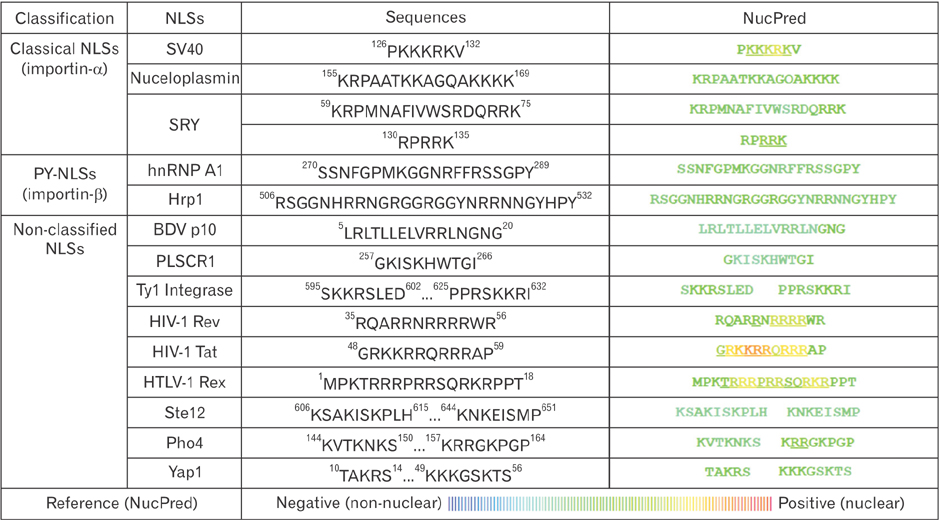
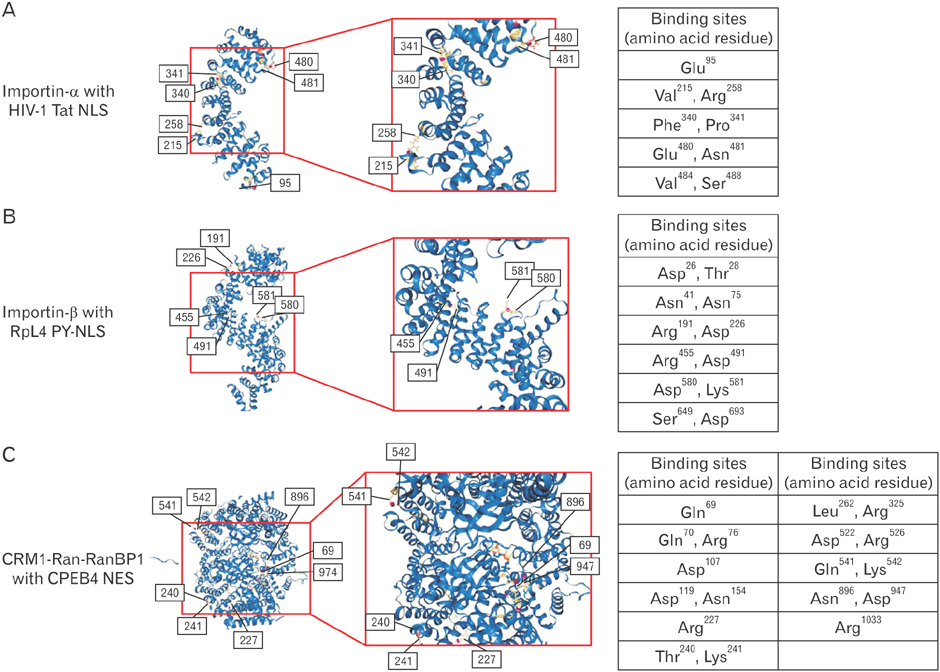
- Transportation between the cytoplasm and the nucleoplasm is critical for many physiological and pathophysiological processes including gene expression, signal transduction, and oncogenesis. So, the molecular mechanism for the transportation needs to be studied not only to understand cell physiological processes but also to develop new diagnostic and therapeutic targets. Recent progress in the research of the nuclear transportation (import and export) via nuclear pore complex and four important factors affecting nuclear transport (nucleoporins, Ran, karyopherins, and nuclear localization signals/nuclear export signals) will be discussed. Moreover, the clinical significance of nuclear transport and its application will be reviewed. This review will provide some critical insight for the molecular design of therapeutics which need to be targeted inside the nucleus.
MeSH Terms
Figure
Reference
-
1. Cautain B, Hill R, de Pedro N, Link W. Components and regulation of nuclear transport processes. FEBS J. 2015; 282:445–462.2. Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004; 4:106–117.3. Bakhoum SF, Compton DA. Chromosomal instability and cancer: a complex relationship with therapeutic potential. J Clin Invest. 2012; 122:1138–1143.4. McLane LM, Corbett AH. Nuclear localization signals and human disease. IUBMB Life. 2009; 61:697–706.5. Patel VP, Chu CT. Nuclear transport, oxidative stress, and neurodegeneration. Int J Clin Exp Pathol. 2011; 4:215–229.6. Hung MC, Link W. Protein localization in disease and therapy. J Cell Sci. 2011; 124(Pt 20):3381–3392.7. Chook YM, Süel KE. Nuclear import by karyopherin-betas: recognition and inhibition. Biochim Biophys Acta. 2011; 1813:1593–1606.8. Chook YM, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001; 11:703–715.9. Conti E, Izaurralde E. Nucleocytoplasmic transport enters the atomic age. Curr Opin Cell Biol. 2001; 13:310–319.10. Görlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999; 15:607–660.11. Tran EJ, Bolger TA, Wente SR. SnapShot: nuclear transport. Cell. 2007; 131:420.12. Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003; 112:441–451.13. Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010; 2:a000562.14. Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007; 8:761–773.15. Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007; 8:195–208.16. Turner JG, Dawson J, Cubitt CL, Baz R, Sullivan DM. Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol. 2014; 27:62–73.17. Wolff T, Unterstab G, Heins G, Richt JA, Kann M. Characterization of an unusual importin alpha binding motif in the borna disease virus p10 protein that directs nuclear import. J Biol Chem. 2002; 277:12151–12157.18. Zolotukhin AS, Felber BK. Nucleoporins nup98 and nup214 participate in nuclear export of human immunodeficiency virus type 1 Rev. J Virol. 1999; 73:120–127.19. Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci U S A. 1999; 96:9112–9117.20. Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998; 242:540–547.21. Cansizoglu AE, Lee BJ, Zhang ZC, Fontoura BM, Chook YM. Structure-based design of a pathway-specific nuclear import inhibitor. Nat Struct Mol Biol. 2007; 14:452–454.22. Carmody SR, Wente SR. mRNA nuclear export at a glance. J Cell Sci. 2009; 122(Pt 12):1933–1937.23. Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997; 90:1051–1060.24. Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, Fransen J, Grosveld G. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997; 16:807–816.25. Imasaki T, Shimizu T, Hashimoto H, Hidaka Y, Kose S, Imamoto N, Yamada M, Sato M. Structural basis for substrate recognition and dissociation by human transportin 1. Mol Cell. 2007; 28:57–67.26. Ohno M, Segref A, Bachi A, Wilm M, Mattaj IW. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell. 2000; 101:187–198.27. Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997; 278:141–144.28. Rodriguez MS, Dargemont C, Stutz F. Nuclear export of RNA. Biol Cell. 2004; 96:639–655.29. Simos G, Grosshans H, Hurt E. Nuclear export of tRNA. Results Probl Cell Differ. 2002; 35:115–131.30. Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997; 90:1041–1050.31. Südbeck P, Scherer G. Two independent nuclear localization signals are present in the DNA-binding high-mobility group domains of SRY and SOX9. J Biol Chem. 1997; 272:27848–27852.32. Tran EJ, King MC, Corbett AH. Macromolecular transport between the nucleus and the cytoplasm: advances in mechanism and emerging links to disease. Biochim Biophys Acta. 2014; 1843:2784–2795.33. Conti E, Uy M, Leighton L, Blobel G, Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell. 1998; 94:193–204.34. Dingwall C, Laskey RA. Nuclear targeting sequences: a consensus? Trends Biochem Sci. 1991; 16:478–481.35. Lee BJ, Cansizoglu AE, Süel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell. 2006; 126:543–558.36. Conti E, Müller CW, Stewart M. Karyopherin flexibility in nucleocytoplasmic transport. Curr Opin Struct Biol. 2006; 16:237–244.37. Cook A, Fernandez E, Lindner D, Ebert J, Schlenstedt G, Conti E. The structure of the nuclear export receptor Cse1 in its cytosolic state reveals a closed conformation incompatible with cargo binding. Mol Cell. 2005; 18:355–367.38. Conti E, Kuriyan J. Crystallographic analysis of the specific yet versatile recognition of distinct nuclear localization signals by karyopherin alpha. Structure. 2000; 8:329–338.39. Isoyama T, Murayama A, Nomoto A, Kuge S. Nuclear import of the yeast AP-1-like transcription factor Yap1p is mediated by transport receptor Pse1p, and this import step is not affected by oxidative stress. J Biol Chem. 2001; 276:21863–21869.40. Kaffman A, Rank NM, O'Shea EK. Phosphorylation regulates association of the transcription factor Pho4 with its import receptor Pse1/Kap121. Genes Dev. 1998; 12:2673–2683.41. Hing ZA, Fung HY, Ranganathan P, Mitchell S, El-Gamal D, Woyach JA, Williams K, Goettl VM, Smith J, Yu X, Meng X, Sun Q, Cagatay T, Lehman AM, Lucas DM, Baloglu E, Shacham S, Kauffman MG, Byrd JC, Chook YM, Garzon R, Lapalombella R. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 2016; 30:2364–2372.42. Huber FM, Hoelz A. Molecular basis for protection of ribosomal protein L4 from cellular degradation. Nat Commun. 2017; 8:14354.43. Rose AS, Bradley AR, Valasatava Y, Duarte JM, Prlić A, Rose PW. Web-based molecular graphics for large complexes. In : Proceedings of the 21st International Conference on Web3D Technolog; 2016 Jul 22-24; Anaheim, California. New York: ACM;2016. p. 185–186.44. Rose AS, Hildebrand PW. NGL Viewer: a web application for molecular visualization. Nucleic Acids Res. 2015; 43:W576–W579.45. Forwood JK, Harley V, Jans DA. The C-terminal nuclear localization signal of the sex-determining region Y (SRY) high mobility group domain mediates nuclear import through importin beta 1. J Biol Chem. 2001; 276:46575–46582.46. Kalderon D, Richardson WD, Markham AF, Smith AE. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984; 311:33–38.47. Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984; 39(3 Pt 2):499–509.48. Poulat F, Girard F, Chevron MP, Gozé C, Rebillard X, Calas B, Lamb N, Berta P. Nuclear localization of the testis determining gene product SRY. J Cell Biol. 1995; 128:737–748.49. Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991; 64:615–623.50. Lange A, Mills RE, Devine SE, Corbett AH. A PY-NLS nuclear targeting signal is required for nuclear localization and function of the Saccharomyces cerevisiae mRNA-binding protein Hrp1. J Biol Chem. 2008; 283:12926–12934.51. Soniat M, Chook YM. Nuclear localization signals for four distinct karyopherin-beta nuclear import systems. Biochem J. 2015; 468:353–362.52. Kobayashi J, Matsuura Y. Structural basis for cell-cycle-dependent nuclear import mediated by the karyopherin Kap121p. J Mol Biol. 2013; 425:1852–1868.53. Maertens GN, Cook NJ, Wang W, Hare S, Gupta SS, Öztop I, Lee K, Pye VE, Cosnefroy O, Snijders AP, KewalRamani VN, Fassati A, Engelman A, Cherepanov P. Structural basis for nuclear import of splicing factors by human Transportin 3. Proc Natl Acad Sci U S A. 2014; 111:2728–2733.54. Chen MH, Ben-Efraim I, Mitrousis G, Walker-Kopp N, Sims PJ, Cingolani G. Phospholipid scramblase 1 contains a nonclassical nuclear localization signal with unique binding site in importin alpha. J Biol Chem. 2005; 280:10599–10606.55. Leslie DM, Grill B, Rout MP, Wozniak RW, Aitchison JD. Kap121p-mediated nuclear import is required for mating and cellular differentiation in yeast. Mol Cell Biol. 2002; 22:2544–2555.56. Moye-Rowley WS, Harshman KD, Parker CS. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989; 3:283–292.57. Roberts RL, Fink GR. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994; 8:2974–2985.58. Truant R, Cullen BR. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol. 1999; 19:1210–1217.59. Eguchi A, Furusawa H, Yamamoto A, Akuta T, Hasegawa M, Okahata Y, Nakanishi M. Optimization of nuclear localization signal for nuclear transport of DNA-encapsulating particles. J Control Release. 2005; 104:507–519.60. Sun Y, Xian L, Xing H, Yu J, Yang Z, Yang T, Yang L, Ding P. Factors influencing the nuclear targeting ability of nuclear localization signals. J Drug Target. 2016; 24:927–933.61. Kim BK, Kang H, Doh KO, Lee SH, Park JW, Lee SJ, Lee TJ. Homodimeric SV40 NLS peptide formed by disulfide bond as enhancer for gene delivery. Bioorg Med Chem Lett. 2012; 22:5415–5418.62. Mahipal A, Malafa M. Importins and exportins as therapeutic targets in cancer. Pharmacol Ther. 2016; 164:135–143.63. Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010; 68:1067–1081.64. Mendes HF, van der Spuy J, Chapple JP, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med. 2005; 11:177–185.65. Mizutani A, Matsuzaki A, Momoi MY, Fujita E, Tanabe Y, Momoi T. Intracellular distribution of a speech/language disorder associated FOXP2 mutant. Biochem Biophys Res Commun. 2007; 353:869–874.66. Sabherwal N, Schneider KU, Blaschke RJ, Marchini A, Rappold G. Impairment of SHOX nuclear localization as a cause for Leri-Weill syndrome. J Cell Sci. 2004; 117(Pt 14):3041–3048.67. Shoubridge C, Tan MH, Fullston T, Cloosterman D, Coman D, McGillivray G, Mancini GM, Kleefstra T, Gécz J. Mutations in the nuclear localization sequence of the Aristaless related homeobox: sequestration of mutant ARX with IPO13 disrupts normal subcellular distribution of the transcription factor and retards cell division. Pathogenetics. 2010; 3:1.68. Wilson GL, Dean BS, Wang G, Dean DA. Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences. J Biol Chem. 1999; 274:22025–22032.69. Kaiser FJ, Brega P, Raff ML, Byers PH, Gallati S, Kay TT, de Almeida S, Horsthemke B, Lüdecke HJ. Novel missense mutations in the TRPS1 transcription factor define the nuclear localization signal. Eur J Hum Genet. 2004; 12:121–126.70. Escriou V, Carrière M, Scherman D, Wils P. NLS bioconjugates for targeting therapeutic genes to the nucleus. Adv Drug Deliv Rev. 2003; 55:295–306.71. Ramakrishna S, Kwaku Dad AB, Beloor J, Gopalappa R, Lee SK, Kim H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014; 24:1020–1027.72. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013; 339:819–823.73. Ma S, Chang J, Wang X, Liu Y, Zhang J, Lu W, Gao J, Shi R, Zhao P, Xia Q. CRISPR/Cas9 mediated multiplex genome editing and heritable mutagenesis of BmKu70 in Bombyx mori. Sci Rep. 2014; 4:4489.74. Wang HY, Chen JX, Sun YX, Deng JZ, Li C, Zhang XZ, Zhuo RX. Construction of cell penetrating peptide vectors with N-terminal stearylated nuclear localization signal for targeted delivery of DNA into the cell nuclei. J Control Release. 2011; 155:26–33.75. Yi WJ, Yang J, Li C, Wang HY, Liu CW, Tao L, Cheng SX, Zhuo RX, Zhang XZ. Enhanced nuclear import and transfection efficiency of TAT peptide-based gene delivery systems modified by additional nuclear localization signals. Bioconjug Chem. 2012; 23:125–134.76. Weisbart RH, Chan G, Jordaan G, Noble PW, Liu Y, Glazer PM, Nishimura RN, Hansen JE. DNA-dependent targeting of cell nuclei by a lupus autoantibody. Sci Rep. 2015; 5:12022.77. Weisbart RH, Gera JF, Chan G, Hansen JE, Li E, Cloninger C, Levine AJ, Nishimura RN. A cell-penetrating bispecific antibody for therapeutic regulation of intracellular targets. Mol Cancer Ther. 2012; 11:2169–2173.78. Brameier M, Krings A, MacCallum RM. NucPred: predicting nuclear localization of proteins. Bioinformatics. 2007; 23:1159–1160.79. Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000; 300:1005–1016.80. Kosugi S, Hasebe M, Tomita M, Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc Natl Acad Sci U S A. 2009; 106:10171–10176.81. la Cour T, Gupta R, Rapacki K, Skriver K, Poulsen FM, Brunak S. NESbase version 1.0: a database of nuclear export signals. Nucleic Acids Res. 2003; 31:393–396.82. Nair R, Carter P, Rost B. NLSdb: database of nuclear localization signals. Nucleic Acids Res. 2003; 31:397–399.83. Nair R, Rost B. Mimicking cellular sorting improves prediction of subcellular localization. J Mol Biol. 2005; 348:85–100.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep Learning in Nuclear Medicine and Molecular Imaging: Current Perspectives and Future Directions
- Medical Application of Radiation Internal Dosimetry
- Use of Nuclear Medicine Technology for Clinical Molecular Imaging: a Message from the Associate Editor
- On Launching a New Twenty-first Century Quarterly Journal, Nuclear Medicine and Molecular Imaging
- Dissecting the molecular mechanism of nuclear receptor action: transcription coactivators and corepressors