Ann Lab Med.
2019 Nov;39(6):572-576. 10.3343/alm.2019.39.6.572.
EDTA Treatment for Overcoming the Prozone Effect and for Predicting C1q Binding in HLA Antibody Testing
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. ejoh@catholic.ac.kr
- KMID: 2450953
- DOI: http://doi.org/10.3343/alm.2019.39.6.572
Abstract
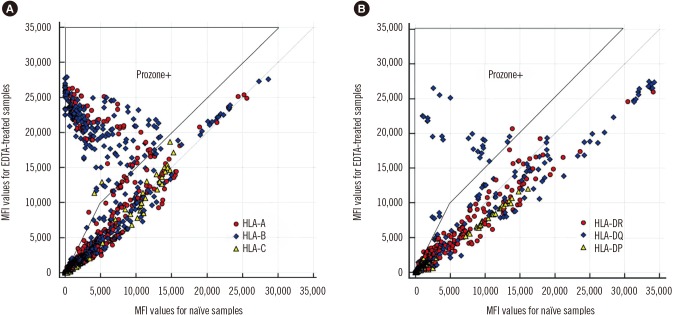
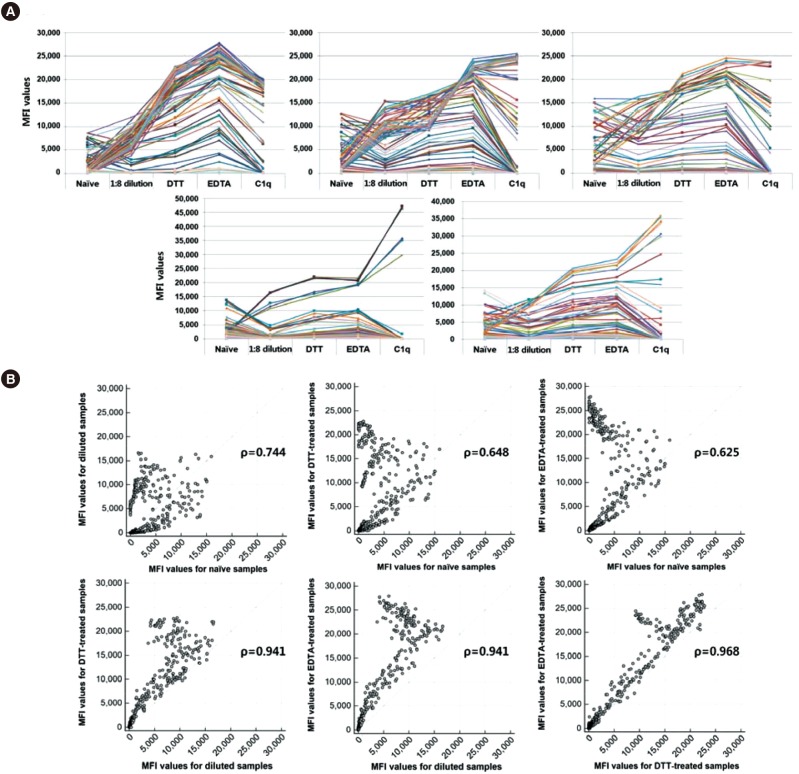
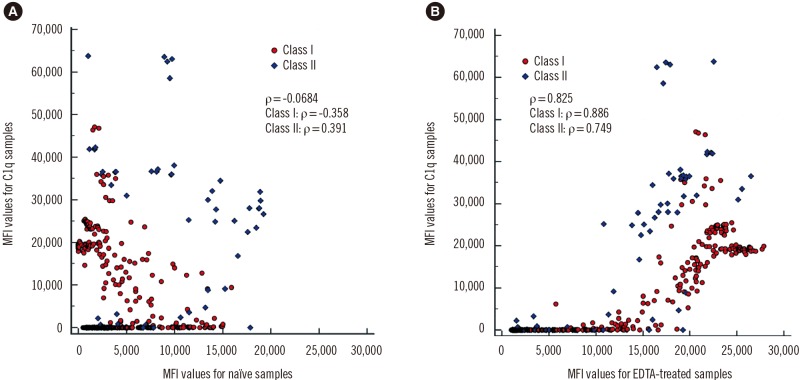
- The Luminex-based single antigen bead (SAB) assay is widely used to detect HLA antibody in transplant recipients. However, one limitation of the SAB assay is the prozone effect, which occurs mostly as a result of complement interference. We investigated the efficacy of EDTA treatment for overcoming the prozone effect and predicting C1q binding of HLA antibody. We subjected 27 non-treated (naïve) and EDTA-treated serum samples from highly sensitized patients to IgG-SAB assays, and we confirmed the prozone effect in 53% and 31% of class I and class II antibody tests, respectively, after EDTA treatment. When we conducted additional assays after dithiothreitol treatment and serum dilution, EDTA was the most efficacious in eliminating the prozone effect. Reducing the prozone effect by EDTA treatment strengthened the correlation between IgG mean fluorescence intensity (MFI) and C1q MFI values (Ï=0.825) as compared with the naïve sera (Ï=0.068). Although C1q positivity was dependent on the concentration of HLA antibody in EDTA-treated sera, the correlations varied individually. Overall, our results confirmed the efficacy of EDTA treatment for overcoming the prozone effect. EDTA treatment showed a positive effect on the correlation between IgG MFI and C1q MFI values.
MeSH Terms
Figure
Cited by 1 articles
-
Causes of Positive Pretransplant Crossmatches in the Absence of Donor-Specific Anti-Human Leukocyte Antigen Antibodies: A Single-Center Experience
Hyunhye Kang, Jaeeun Yoo, Sang-Yoon Lee, Eun-Jee Oh
Ann Lab Med. 2021;41(4):429-435. doi: 10.3343/alm.2021.41.4.429.
Reference
-
1. Tambur AR, Campbell P, Claas FH, Feng S, Gebel HM, Jackson AM, et al. Sensitization in transplantation: assessment of risk (STAR) 2017 working group meeting report. Am J Transplant. 2018; 18:1604–1614. PMID: 29603613.2. Oh EJ, Park H, Park KU, Kang ES, Kim HS, Song EY. Interlaboratory comparison of the results of Lifecodes LSA Class I and Class II Single Antigen Kits for Human Leukocyte Antigen Antibody Detection. Ann Lab Med. 2015; 35:321–328. PMID: 25932440.3. Gebel HM, Bray RA. The evolution and clinical impact of human leukocyte antigen technology. Curr Opin Nephrol Hypertens. 2010; 19:598–602. PMID: 20720491.4. Jang JY, Kim YJ, Kim Y, Park YJ, Han K, Oh EJ. Application of calculated panel reactive antibody using HLA frequencies in Koreans. Ann Lab Med. 2012; 32:66–72. PMID: 22259781.5. Min JW, Lee H, Choi BS, Park CW, Yang CW, Kim YS, et al. Clinical impact of pre-transplant antibodies against angiotensin II Type I receptor and major histocompatibility complex class I-related chain A in kidney transplant patients. Ann Lab Med. 2018; 38:450–457. PMID: 29797816.6. Mujtaba MA, Goggins W, Lobashevsky A, Sharfuddin AA, Yaqub MS, Mishler DP, et al. The strength of donor-specific antibody is a more reliable predictor of antibody-mediated rejection than flow cytometry crossmatch analysis in desensitized kidney recipients. Clin Transplant. 2011; 25:E96–E102. PMID: 20977497.7. Zachary A, Reinsmoen NL. Quantifying HLA-specific antibodies in patients undergoing desensitization. Curr Opin Organ Transplant. 2011; 16:410–415. PMID: 21666475.8. Lee H, Han E, Choi AR, Ban TH, Chung BH, Yang CW, et al. Clinical impact of complement (C1q, C3d) binding de novo donor-specific HLA antibody in kidney transplant recipients. PLoS One. 2018; 13:e0207434. PMID: 30427941.9. Tambur AR. HLA diagnostics: evaluating DSA strength by titration. Transplantation. 2018; 102:S23–S30. PMID: 29266059.10. Tambur AR, Herrera ND, Haarberg KM, Cusick MF, Gordon RA, Leventhal JR, et al. Assessing antibody strength: comparison of MFI, C1q, and titer information. Am J Transplant. 2015; 15:2421–2430. PMID: 25930984.11. Schnaidt M, Weinstock C, Jurisic M, Schmid-Horch B, Ender A, Wernet D. HLA antibody specification using single-antigen beads—a technical solution for the prozone effect. Transplantation. 2011; 92:510–515. PMID: 21869744.12. Zhang X, Reinsmoen NL. Comprehensive assessment for serum treatment for single antigen test for detection of HLA antibodies. Hum Immunol. 2017; 78:699–703. PMID: 28899793.13. Ellis TM. Interpretation of HLA single antigen bead assays. Transplant Rev (Orlando). 2013; 27:108–111. PMID: 23958238.14. Tambur AR, Glotz D, Herrera ND, Chatroop EN, Roitberg T, Friedewald JJ, et al. Can solid phase assays be better utilized to measure efficacy of antibody removal therapies? Hum Immunol. 2016; 77:624–630. PMID: 27267046.15. Kosmoliaptsis V, Bradley JA, Peacock S, Chaudhry AN, Taylor CJ. Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation. 2009; 87:813–820. PMID: 19300182.16. Schwaiger E, Wahrmann M, Bond G, Eskandary F, Böhmig GA. Complement component C3 activation: the leading cause of the prozone phenomenon affecting HLA antibody detection on single-antigen beads. Transplantation. 2014; 97:1279–1285. PMID: 24621535.17. Liu C, Pang S, Phelan D, Brennan DC, Mohanakumar T. Quantitative evaluation of the impact of ethylenediaminetetraacetic acid pretreatment on single-antigen bead assay. Transplant Direct. 2017; 3:e194. PMID: 28795145.18. Guidicelli G, Visentin J, Franchini N, Borg C, Merville P, Couzi L, et al. Prevalence, distribution and amplitude of the complement interference phenomenon in single antigen flow beads assays. HLA. 2018; 91:507–513. PMID: 29604172.19. Hahn AB. Inactivation of IgM antibodies. In : Hahn AB, Land GA, Strothman RM, editors. ASHI laboratory manual I. 4th ed. Mount Laurel: Society for Histocompatibility and Immunogenetics;2000. p. 4.1.20. Taylor CJ, Kosmoliaptsis V, Martin J, Knighton G, Mallon D, Bradley JA, et al. Technical limitations of the C1q Single-Antigen Bead Assay to detect complement binding HLA-specific antibodies. Transplantation. 2017; 101:1206–1214. PMID: 27306532.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical characteristics of antibody-mediated rejections with C1qbinding and without C1-binding donor-specific antibodies
- The clinical utility of preformed C1q-binding donor-specific anti-HLA antibodies in kidney transplantation
- EDTA Inhibits the Binding of Clone 96.2C1, an Anti-CD41a Monoclonal Antibody, to the Platelets and Addition of Heparin and CaCl2 to the Antibody Neutralizes the EDTA-induced Inhibitory Effect
- Serologic heterogeneity of HLA-A24 correlates with allelic types in the Korean population
- Human leukocyte antigen epitope definition using site-directed mutagenesis