Int J Stem Cells.
2019 Mar;12(1):31-42. 10.15283/ijsc18084.
Alteration of Genomic Imprinting Status of Human Parthenogenetic Induced Pluripotent Stem Cells during Neural Lineage Differentiation
- Affiliations
-
- 1Departement of Stem Cell Biology, Konkuk University School of Medicine, Seoul, Korea. knko@kku.ac.kr
- 2Center for Stem Cell Research, Institute of Advanced Biomedical Science, Konkuk University, Seoul, Korea.
- 3Department of Medicine, College of Medicine, Chung-Ang University, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, College of Medicine, Chung-Ang University, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Konkuk University School of Medicine, Seoul, Korea.
- 6Research Institute of Medical Science, Konkuk University, Seoul, Korea.
- KMID: 2447222
- DOI: http://doi.org/10.15283/ijsc18084
Abstract
- BACKGROUND AND OBJECTIVES
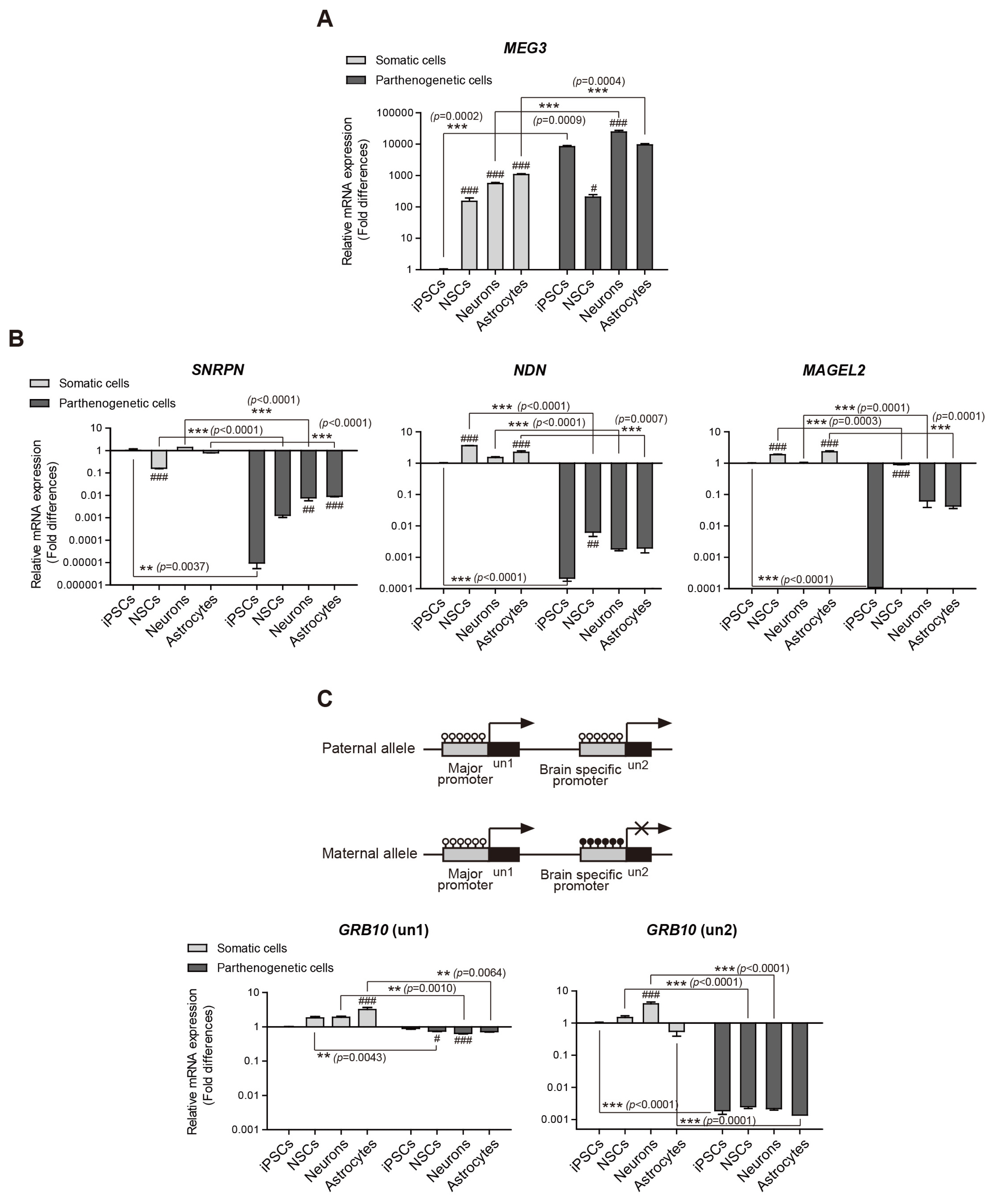
Genomic imprinting modulates growth and development in mammals and is associated with genetic disorders. Although uniparental embryonic stem cells have been used to study genomic imprinting, there is an ethical issue associated with the destruction of human embryos. In this study, to investigate the genomic imprinting status in human neurodevelopment, we used human uniparental induced pluripotent stem cells (iPSCs) that possessed only maternal alleles and differentiated into neural cell lineages.
METHODS
Human somatic iPSCs (hSiPSCs) and human parthenogenetic iPSCs (hPgiPSCs) were differentiated into neural stem cells (NSCs) and named hSi-NSCs and hPgi-NSCs respectively. DNA methylation and gene expression of imprinted genes related neurodevelopment was analyzed during reprogramming and neural lineage differentiation.
RESULTS
The DNA methylation and expression of imprinted genes were altered or maintained after differentiation into NSCs. The imprinting status in NSCs were maintained after terminal differentiation into neurons and astrocytes. In contrast, gene expression was differentially presented in a cell type-specific manner.
CONCLUSIONS
This study suggests that genomic imprinting should be determined in each neural cell type because the genomic imprinting status can differ in a cell type-specific manner. In addition, the in vitro model established in this study would be useful for verifying the epigenetic alteration of imprinted genes which can be differentially changed during neurodevelopment in human and for screening novel imprinted genes related to neurodevelopment. Moreover, the confirmed genomic imprinting status could be used to find out an abnormal genomic imprinting status of imprinted genes related with neurogenetic disorders according to uniparental genotypes.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001; 2:21–32. DOI: 10.1038/35047554. PMID: 11253064.
Article2. Plasschaert RN, Bartolomei MS. Genomic imprinting in development, growth, behavior and stem cells. Development. 2014; 141:1805–1813. DOI: 10.1242/dev.101428. PMID: 24757003. PMCID: 3994769.
Article3. Battaglia A. The inv dup(15) or idic(15) syndrome: a clinically recognisable neurogenetic disorder. Brain Dev. 2005; 27:365–369. DOI: 10.1016/j.braindev.2004.08.006. PMID: 16023554.4. Woodfine K, Huddleston JE, Murrell A. Quantitative analysis of DNA methylation at all human imprinted regions reveals preservation of epigenetic stability in adult somatic tissue. Epigenetics Chromatin. 2011; 4:1. DOI: 10.1186/1756-8935-4-1. PMID: 21281512. PMCID: 3038880.
Article5. Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010; 329:643–648. DOI: 10.1126/science.1190830. PMID: 20616232. PMCID: 3005244.
Article6. Caspary T, Cleary MA, Perlman EJ, Zhang P, Elledge SJ, Tilghman SM. Oppositely imprinted genes p57(Kip2) and igf2 interact in a mouse model for Beckwith-Wiedemann syndrome. Genes Dev. 1999; 13:3115–3124. DOI: 10.1101/gad.13.23.3115. PMID: 10601037. PMCID: 317182.
Article7. Epsztejn-Litman S, Cohen-Hadad Y, Aharoni S, Altarescu G, Renbaum P, Levy-Lahad E, et al. Establishment of homozygote mutant human embryonic stem cells by parthenogenesis. PLoS One. 2015; 10:e0138893. DOI: 10.1371/journal.pone.0138893. PMID: 26473610. PMCID: 4608834.
Article8. Stelzer Y, Bar S, Bartok O, Afik S, Ronen D, Kadener S, Benvenisty N. Differentiation of human parthenogenetic pluripotent stem cells reveals multiple tissue- and isoform-specific imprinted transcripts. Cell Rep. 2015; 11:308–320. DOI: 10.1016/j.celrep.2015.03.023. PMID: 25843718.
Article9. Stelzer Y, Ronen D, Bock C, Boyle P, Meissner A, Benvenisty N. Identification of novel imprinted differentially methylated regions by global analysis of human-parthenogenetic-induced pluripotent stem cells. Stem Cell Reports. 2013; 1:79–89. DOI: 10.1016/j.stemcr.2013.03.005. PMID: 24052944. PMCID: 3757747.
Article10. Stelzer Y, Yanuka O, Benvenisty N. Global analysis of parental imprinting in human parthenogenetic induced pluripotent stem cells. Nat Struct Mol Biol. 2011; 18:735–741. DOI: 10.1038/nsmb.2050. PMID: 21572443.
Article11. Choi NY, Bang JS, Lee HJ, Park YS, Lee M, Jeong D, Ko K, Han DW, Chung HM, Kim GJ, Shim SH, Hwang HS, Ko K. Novel imprinted single CpG sites found by global DNA methylation analysis in human parthenogenetic induced pluripotent stem cells. Epigenetics. 2018; 13:343–351. DOI: 10.1080/15592294.2018.1460033. PMID: 29613829. PMCID: 6140819.
Article12. Mo WM, Kwon YW, Jang IH3, Choi EJ, Kwon SM, Kim JH. Role of TAZ in lysophosphatidic acid-induced migration and proliferation of human adipose-derived mesenchymal stem cells. Biomol Ther (Seoul). 2017; 25:354–361. DOI: 10.4062/biomolther.2016.263. PMID: 28554198. PMCID: 5499612.
Article13. Lee HJ, Choi NY, Lee SW, Ko K, Hwang TS, Han DW, Lim J, Schöler HR, Ko K. Epigenetic alteration of imprinted genes during neural differentiation of germline-derived pluripotent stem cells. Epigenetics. 2016; 11:177–183. DOI: 10.1080/15592294.2016.1146852. PMID: 26962997. PMCID: 4854545.
Article14. Chamberlain SJ, Chen PF, Ng KY, Bourgois-Rocha F, Lemtiri-Chlieh F, Levine ES, Lalande M. Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc Natl Acad Sci U S A. 2010; 107:17668–17673. DOI: 10.1073/pnas.1004487107. PMID: 20876107. PMCID: 2955112.
Article15. Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell. 2016; 18:736–748. DOI: 10.1016/j.stem.2016.05.022. PMID: 27257762.
Article16. Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schübeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007; 39:457–466. DOI: 10.1038/ng1990. PMID: 17334365.
Article17. Burnett LC, LeDuc CA, Sulsona CR, Paull D, Eddiry S, Levy B, Salles JP, Tauber M, Driscoll DJ, Egli D, Leibel RL. Induced pluripotent stem cells (iPSC) created from skin fibroblasts of patients with Prader-Willi syndrome (PWS) retain the molecular signature of PWS. Stem Cell Res. 2016; 17:526–530. DOI: 10.1016/j.scr.2016.08.008. PMID: 27789403.
Article18. Lau JC, Hanel ML, Wevrick R. Tissue-specific and imprinted epigenetic modifications of the human NDN gene. Nucleic Acids Res. 2004; 32:3376–3382. DOI: 10.1093/nar/gkh671. PMID: 15247330. PMCID: 443546.
Article19. Hippenmeyer S, Johnson RL, Luo L. Mosaic analysis with double markers reveals cell-type-specific paternal growth dominance. Cell Rep. 2013; 3:960–967. DOI: 10.1016/j.celrep.2013.02.002. PMID: 23453967. PMCID: 3668097.
Article20. Hikichi T, Kohda T, Kaneko-Ishino T, Ishino F. Imprinting regulation of the murine Meg1/Grb10 and human GRB10 genes; roles of brain-specific promoters and mouse-specific CTCF-binding sites. Nucleic Acids Res. 2003; 31:1398–1406. DOI: 10.1093/nar/gkg232. PMID: 12595547. PMCID: 149825.
Article21. Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer. 2011; 129:773–779. DOI: 10.1002/ijc.26052. PMID: 21400503.
Article22. Nishino K, Toyoda M, Yamazaki-Inoue M, Fukawatase Y, Chikazawa E, Sakaguchi H, Akutsu H, Umezawa A. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet. 2011; 7:e1002085. DOI: 10.1371/journal.pgen.1002085. PMID: 21637780. PMCID: 3102737.
Article23. Lee S, Kozlov S, Hernandez L, Chamberlain SJ, Brannan CI, Stewart CL, Wevrick R. Expression and imprinting of MAGEL2 suggest a role in Prader-willi syndrome and the homologous murine imprinting phenotype. Hum Mol Genet. 2000; 9:1813–1819. DOI: 10.1093/hmg/9.12.1813. PMID: 10915770.
Article24. Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet. 2012; 28:33–42. DOI: 10.1016/j.tig.2011.09.004. PMID: 22019337.
Article25. Brant JO, Riva A, Resnick JL, Yang TP. Influence of the Prader-Willi syndrome imprinting center on the DNA methylation landscape in the mouse brain. Epigenetics. 2014; 9:1540–1556. DOI: 10.4161/15592294.2014.969667. PMID: 25482058. PMCID: 4623435.
Article26. Adalsteinsson BT, Ferguson-Smith AC. Epigenetic control of the genome-lessons from genomic imprinting. Genes (Basel). 2014; 5:635–655. DOI: 10.3390/genes5030635. PMID: 25257202. PMCID: 4198922.
Article27. Takahashi N, Okamoto A, Kobayashi R, Shirai M, Obata Y, Ogawa H, Sotomaru Y, Kono T. Deletion of Gtl2, imprinted non-coding RNA, with its differentially methylated region induces lethal parent-origin-dependent defects in mice. Hum Mol Genet. 2009; 18:1879–1888. DOI: 10.1093/hmg/ddp108. PMID: 19264764.
Article28. Horsthemke B, Wagstaff J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am J Med Genet A. 2008; 146A:2041–2052. DOI: 10.1002/ajmg.a.32364. PMID: 18627066.
Article29. Matarazzo V, Muscatelli F. Natural breaking of the maternal silence at the mouse and human imprinted Prader-Willi locus: a whisper with functional consequences. Rare Dis. 2013; 1:e27228. DOI: 10.4161/rdis.27228. PMID: 25003016. PMCID: 3978896.30. Yamasaki-Ishizaki Y, Kayashima T, Mapendano CK, Soejima H, Ohta T, Masuzaki H, Kinoshita A, Urano T, Yoshiura K, Matsumoto N, Ishimaru T, Mukai T, Niikawa N, Kishino T. Role of DNA methylation and histone H3 lysine 27 methylation in tissue-specific imprinting of mouse Grb10. Mol Cell Biol. 2007; 27:732–742. DOI: 10.1128/MCB.01329-06. PMID: 17101788. PMCID: 1800802.
Article31. Cheon CK. Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Ann Pediatr Endocrinol Metab. 2016; 21:126–135. DOI: 10.6065/apem.2016.21.3.126. PMID: 27777904. PMCID: 5073158.
Article32. Chamberlain SJ, Lalande M. Angelman syndrome, a genomic imprinting disorder of the brain. J Neurosci. 2010; 30:9958–9963. DOI: 10.1523/JNEUROSCI.1728-10.2010. PMID: 20668179.
Article33. Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997; 15:70–73. DOI: 10.1038/ng0197-70. PMID: 8988171.
Article34. Stanurova J, Neureiter A, Hiber M, de Oliveira Kessler H, Stolp K, Goetzke R, Klein D, Bankfalvi A, Klump H, Steenpass L. Angelman syndrome-derived neurons display late onset of paternal UBE3A silencing. Sci Rep. 2016; 6:30792. DOI: 10.1038/srep30792. PMID: 27484051. PMCID: 4971516.
Article35. Grier MD, Carson RP, Lagrange AH. Toward a broader view of Ube3a in a mouse model of angelman syndrome: expression in brain, spinal cord, sciatic nerve and glial cells. PLoS One. 2015; 10:e0124649. DOI: 10.1371/journal.pone.0124649. PMID: 25894543. PMCID: 4403805.
Article36. Judson MC, Sosa-Pagan JO, Del Cid WA, Han JE, Philpot BD. Allelic specificity of Ube3a expression in the mouse brain during postnatal development. J Comp Neurol. 2014; 522:1874–1896. DOI: 10.1002/cne.23507. PMID: 24254964. PMCID: 3984624.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcriptional Profiles of Imprinted Genes in Human Embryonic Stem Cells During In vitro Differentiation
- Human Placenta-Derived ECM Supports Tri-Lineage Differentiation of Human Induced Pluripotent Stem Cells
- New Advances in Human X Chromosome Status from a Developmental and Stem Cell Biology
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- Regulation of Neural Stem Cell Fate by Natural Products