J Dent Anesth Pain Med.
2019 Apr;19(2):91-99. 10.17245/jdapm.2019.19.2.91.
Remifentanil promotes osteoblastogenesis by upregulating Runx2/osterix expression in preosteoblastic C2C12 cells
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Yangsan, Korea. kejdream84@naver.com
- 2Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea.
- 3Department of Oral Physiology, School of Dentistry, Pusan National University, Yangsan, Korea.
- KMID: 2444753
- DOI: http://doi.org/10.17245/jdapm.2019.19.2.91
Abstract
- BACKGROUND
The imbalance between osteoblasts and osteoclasts can lead to pathological conditions such as osteoporosis. It has been reported that opioid adversely affect the skeletal system, but it is inconsistent. Remifentanil is currently used as an adjuvant analgesic drug in general anesthesia and sedation. The aim of the present study was to investigate the effect of remifentanil on the osteoblast differentiation and mechanism involved in this effect.
METHODS
The C2C12 cells (mouse pluripotent mesenchymal cell line) were used as preosteoblast. Osteoblastic differentiation potency was determined by alkaline phosphatase (ALP) staining. C2C12 cell migration by remifentanil was evaluated using Boyden chamber migration assay. The expression of Runx2 and osterix was evaluated by RT-PCT and western blot analysis to investigate the mechanism involved in remifentanil-mediated osteoblast differentiation.
RESULTS
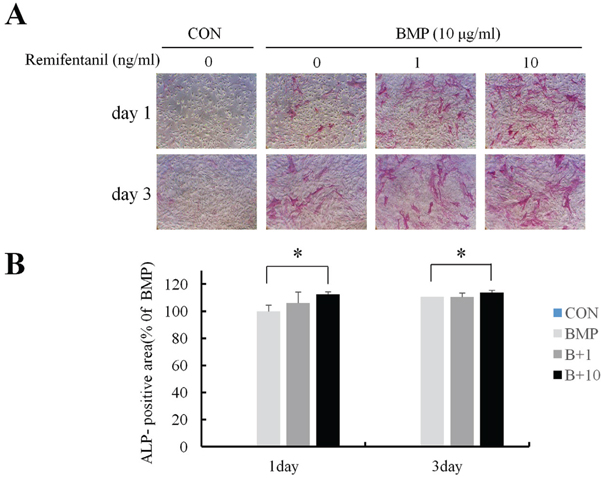
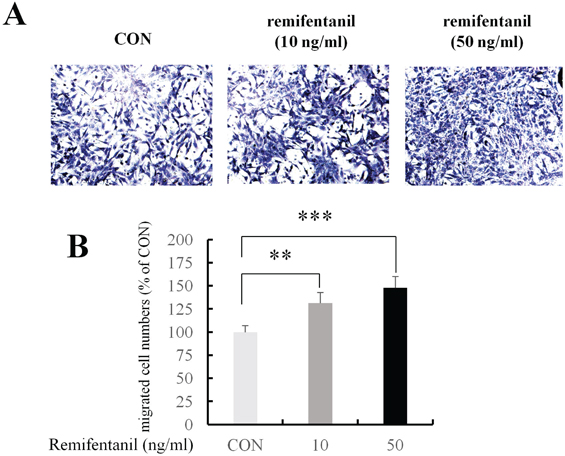
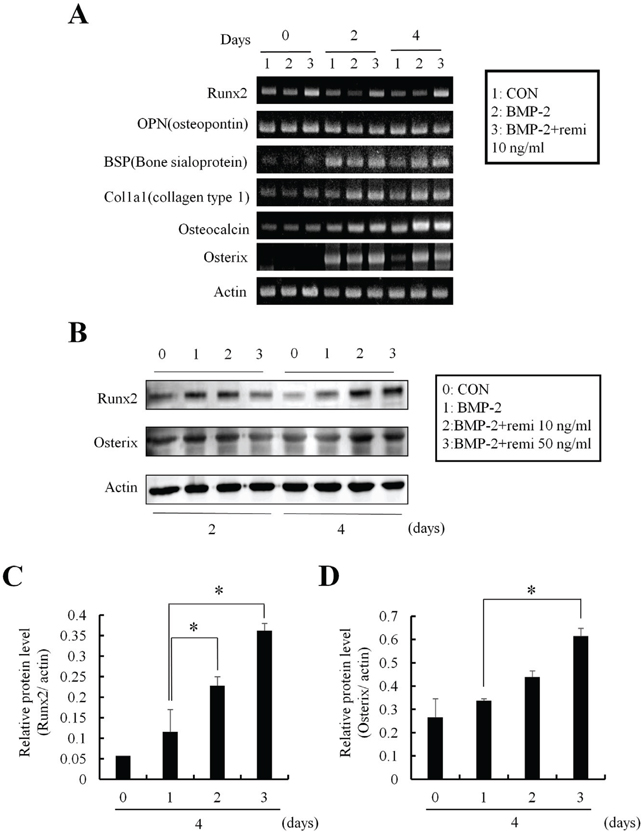
ALP staining showed that remifentanil increased significantly osteoblast differentiation. In Boyden chamber migration assay, C2C12 cell migration was increased by remifentanil. RT-PCR and western blot analysis showed that the expression of Runx2 and osterix was upregulated by remifentanil.
CONCLUSIONS
We demonstrated that remifentanil increased osteoblast differentiation in vitro by upregulation of Runx2 and osterix expression. Therefore, remifentanil has the potential for assisting with bone formation and bone healing.
Keyword
MeSH Terms
Figure
Reference
-
1. Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009; 15:208–216.
Article2. Hill PA. Bone remodelling. Br J Orthod. 1998; 25:101–107.
Article3. Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010; 6:99–105.
Article4. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article5. Jensen ED, Gopalakrishnan R, Westendorf JJ. Regulation of gene expression in osteoblasts. Biofactors. 2010; 36:25–32.
Article6. Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011; 13:27–38.
Article7. Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, et al. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004; 23:4315–4329.
Article8. Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007; 62:1266–1280.
Article9. Yoon JY, Kim DW, Kim EJ, Park BS, Yoon JU, Kim HJ, et al. Protective effects of remifentanil against H2O2-induced oxidative stress in human osteoblasts. J Dent Anesth Pain Med. 2016; 16:263–271.
Article10. Baik SW, Park BS, Kim YH, Kim YD, Kim CH, Yoon JY, et al. Effects of Remifentanil Preconditioning on Osteoblasts under Hypoxia-Reoxygenation Condition. Int J Med Sci. 2015; 12:583–589.
Article11. Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012; 45:863–873.
Article12. Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004; 15:49–60.
Article13. Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994; 127:1755–1766.
Article14. Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013; 6:32–52.
Article15. Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003; 85-A:1544–1552.
Article16. Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002; 277:33361–33368.
Article17. Delaisse JM. The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014; 3:561.
Article18. Dirckx N, Van Hul M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today. 2013; 99:170–191.
Article19. Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009; 15:757–765.
Article20. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002; 108:17–29.
Article21. Nishio Y, Dong Y, Paris M, O'Keefe RJ, Schwarz EM, Drissi H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006; 372:62–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunolocalization of Runx2 and Osterix in the Developing Periodontal Tissues of the Mouse
- Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts
- Aqueous extract of Petasites japonicus leaves promotes osteoblast differentiation via up-regulation of Runx2 and Osterix in MC3T3-E1 cells
- YBX1 Promotes the Inclusion of RUNX2 Alternative Exon 5 in Dental Pulp Stem Cells
- Silencing of LncRNA-ANCR Promotes the Osteogenesis of Osteoblast Cells in Postmenopausal Osteoporosis via Targeting EZH2 and RUNX2