Korean J Physiol Pharmacol.
2019 Jan;23(1):1-20. 10.4196/kjpp.2019.23.1.1.
An integrated review on new targets in the treatment of neuropathic pain
- Affiliations
-
- 1Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala 147002, India. amteshwarjaggi@yahoo.co.in
- 2Akal College of Pharmacy and Technical Education, Mastuana Sahib 148002, Sangrur, India.
- KMID: 2443611
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.1
Abstract
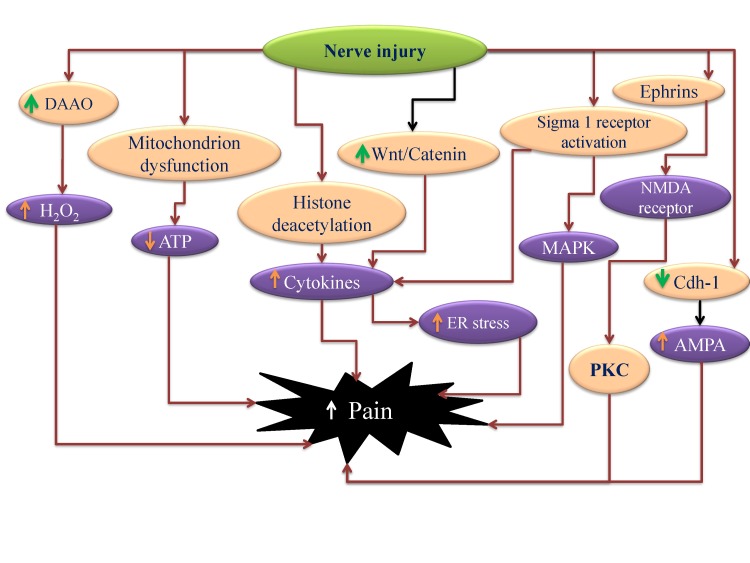
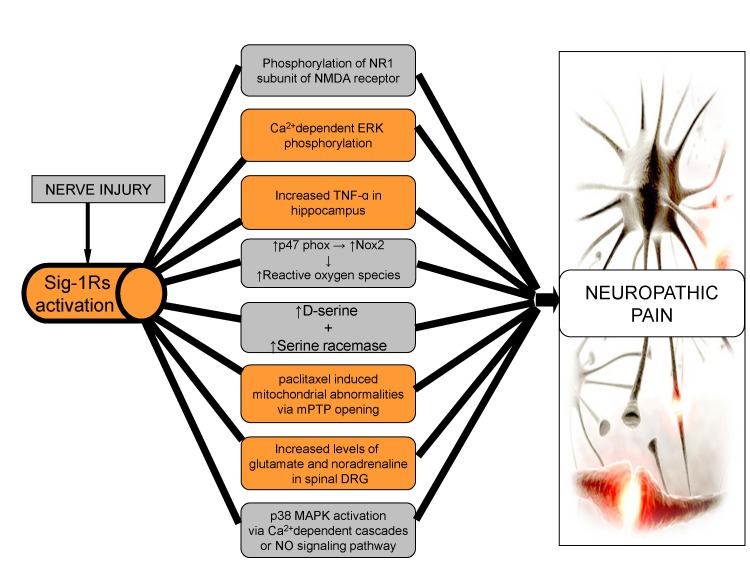
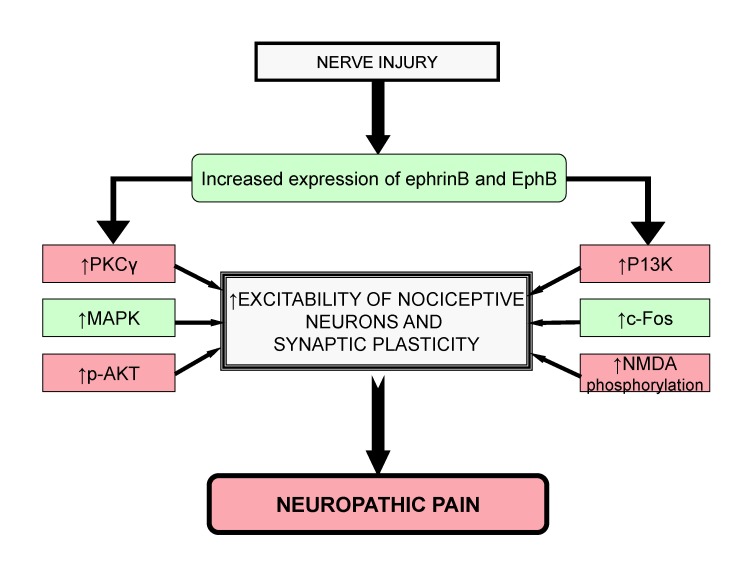
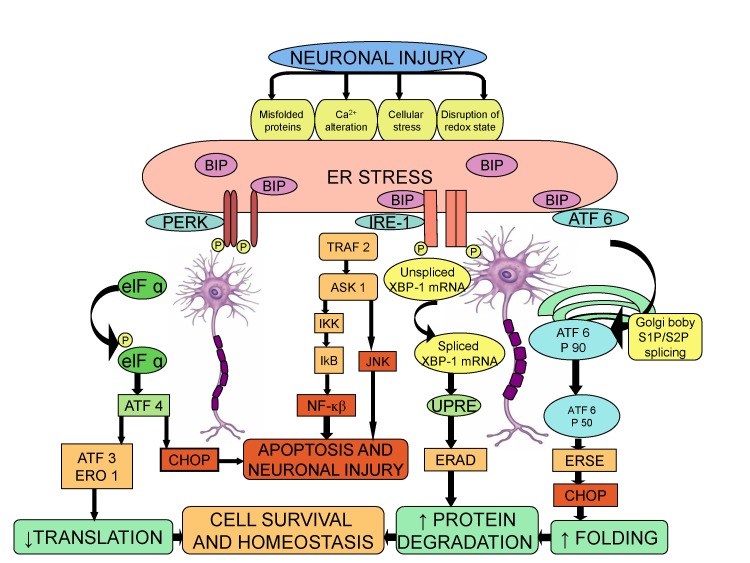
- Neuropathic pain is a complex chronic pain state caused by the dysfunction of somatosensory nervous system, and it affects the millions of people worldwide. At present, there are very few medical treatments available for neuropathic pain management and the intolerable side effects of medications may further worsen the symptoms. Despite the presence of profound knowledge that delineates the pathophysiology and mechanisms leading to neuropathic pain, the unmet clinical needs demand more research in this field that would ultimately assist to ameliorate the pain conditions. Efforts are being made globally to explore and understand the basic molecular mechanisms responsible for somatosensory dysfunction in preclinical pain models. The present review highlights some of the novel molecular targets like D-amino acid oxidase, endoplasmic reticulum stress receptors, sigma receptors, hyperpolarization-activated cyclic nucleotide-gated cation channels, histone deacetylase, Wnt/β-catenin and Wnt/Ryk, ephrins and Eph receptor tyrosine kinase, Cdh-1 and mitochondrial ATPase that are implicated in the induction of neuropathic pain. Studies conducted on the different animal models and observed results have been summarized with an aim to facilitate the efforts made in the drug discovery. The diligent analysis and exploitation of these targets may help in the identification of some promising therapies that can better manage neuropathic pain and improve the health of patients.
Keyword
MeSH Terms
-
Adenosine Triphosphatases
Chronic Pain
Cyclic Nucleotide-Gated Cation Channels
Drug Discovery
Endoplasmic Reticulum Stress
Ephrins
Histone Deacetylases
Humans
Models, Animal
Nervous System
Neuralgia*
Oxidoreductases
Receptors, Eph Family
Receptors, sigma
Adenosine Triphosphatases
Cyclic Nucleotide-Gated Cation Channels
Ephrins
Histone Deacetylases
Oxidoreductases
Receptors, Eph Family
Receptors, sigma
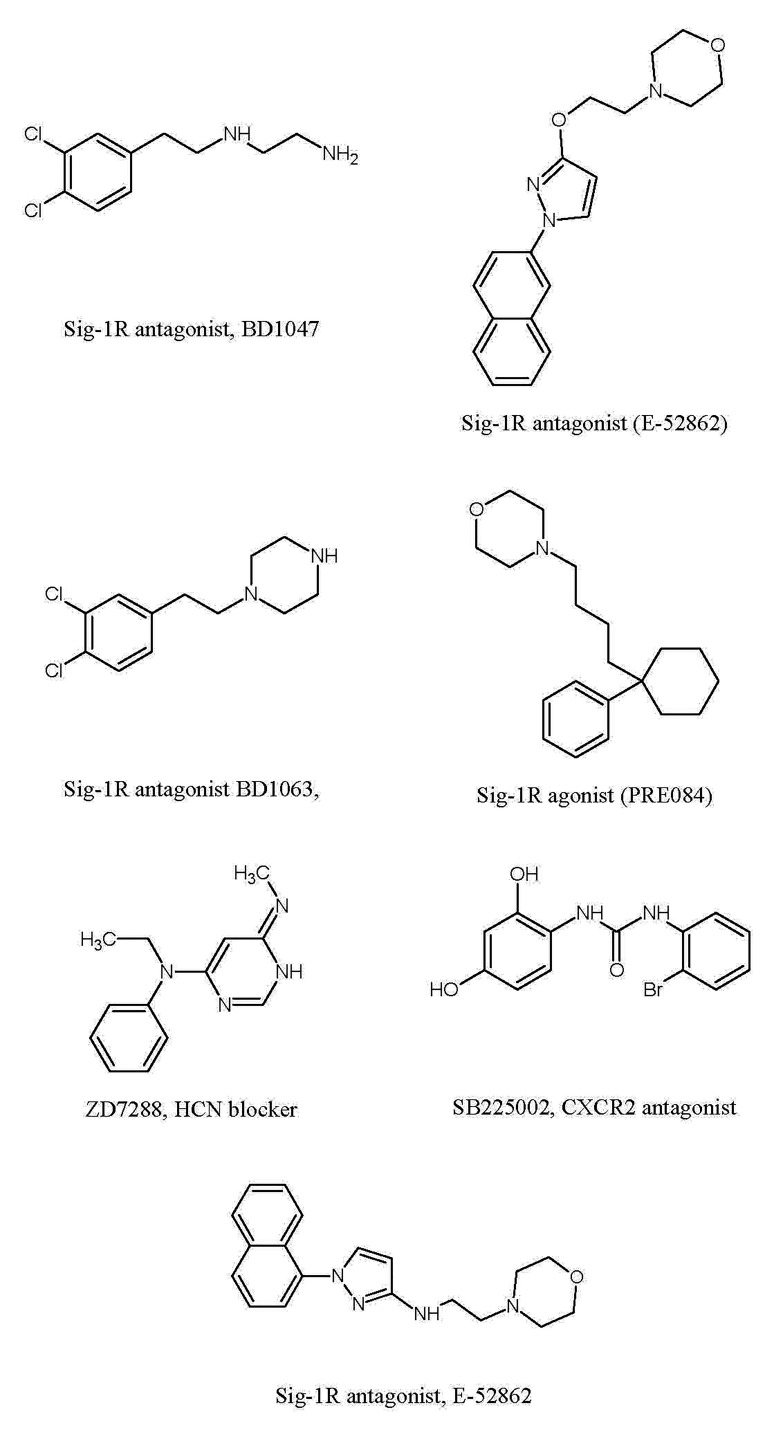
Figure
Reference
-
1. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999; 353:1959–1964. PMID: 10371588.
Article2. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002; 18:343–349. PMID: 12441827.
Article3. Jaggi AS, Singh N. Therapeutic targets for the management of peripheral nerve injury-induced neuropathic pain. CNS Neurol Disord Drug Targets. 2011; 10:589–609. PMID: 21631400.4. Alston RP, Pechon P. Dysaesthesia associated with sternotomy for heart surgery. Br J Anaesth. 2005; 95:153–158. PMID: 15894562.5. Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA, Chandran S, MacLeod MR, Fallon MT. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013; 154:632–642. PMID: 23318126.
Article6. Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009; 374:1252–1261. PMID: 19796802.
Article7. Dunner DL, Wohlreich MM, Mallinckrodt CH, Watkin JG, Fava M. Clinical consequences of initial duloxetine dosing strategies: comparison of 30 and 60 mg QD starting doses. Curr Ther Res Clin Exp. 2005; 66:522–540. PMID: 24678074.
Article8. Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005; 118:289–305. PMID: 16213659.
Article9. Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res. 2013; 36:237–251. PMID: 23435945.
Article10. Verma V, Singh N, Singh Jaggi A. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol. 2014; 12:44–56. PMID: 24533015.
Article11. Muthuraman A, Singh N, Jaggi AS, Ramesh M. Drug therapy of neuropathic pain: current developments and future perspectives. Curr Drug Targets. 2014; 15:210–253. PMID: 24093749.
Article12. Yasuda T, Miki S, Yoshinaga N, Senba E. Effects of amitriptyline and gabapentin on bilateral hyperalgesia observed in an animal model of unilateral axotomy. Pain. 2005; 115:161–170. PMID: 15836979.
Article13. Zuliani V, Rivara M, Fantini M, Costantino G. Sodium channel blockers for neuropathic pain. Expert Opin Ther Pat. 2010; 20:755–779. PMID: 20384535.
Article14. Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol. 2008; 99:3151–3156. PMID: 18417624.
Article15. Levy D, Tal M, Höke A, Zochodne DW. Transient action of the endothelial constitutive nitric oxide synthase (ecNOS) mediates the development of thermal hypersensitivity following peripheral nerve injury. Eur J Neurosci. 2000; 12:2323–2332. PMID: 10947811.
Article16. Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007; 87:659–797. PMID: 17429044.
Article17. Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009; 144:187–199. PMID: 19446956.
Article18. Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008; 139:117–126. PMID: 18442882.
Article19. Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. J Neurophysiol. 2010; 103:2570–2580. PMID: 20220084.
Article20. Roza C, Lopez-Garcia JA. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain. 2008; 138:537–545. PMID: 18331780.
Article21. Lee J, Back SK, Lim EJ, Cho GC, Kim MA, Kim HJ, Lee MH, Na HS. Are spinal GABAergic elements related to the manifestation of neuropathic pain in rat? Korean J Physiol Pharmacol. 2010; 14:59–69. PMID: 20473376.
Article22. Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009; 110:155–165. PMID: 19104183.
Article23. Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004; 45:89–95. PMID: 14648549.
Article24. Sommer C, Lindenlaub T, Teuteberg P, Schäfers M, Hartung T, Toyka KV. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001; 913:86–89. PMID: 11532251.
Article25. Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004; 109:124–131. PMID: 15082134.
Article26. Nabekura J, Ueno T, Okabe A, Furuta A, Iwaki T, Shimizu-Okabe C, Fukuda A, Akaike N. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J Neurosci. 2002; 22:4412–4417. PMID: 12040048.27. Scheff NN, Gold MS. Trafficking of Na+/Ca2+ exchanger to the site of persistent inflammation in nociceptive afferents. J Neurosci. 2015; 35:8423–8432. PMID: 26041911.28. Lee SE, Kim JH. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci Res. 2007; 58:245–249. PMID: 17428562.
Article29. Bannon AW, Decker MW, Kim DJ, Campbell JE, Arneric SP. ABT-594, a novel cholinergic channel modulator, is efficacious in nerve ligation and diabetic neuropathy models of neuropathic pain. Brain Res. 1998; 801:158–163. PMID: 9729357.
Article30. Chen XL, Li XY, Qian SB, Wang YC, Zhang PZ, Zhou XJ, Wang YX. Down-regulation of spinal D-amino acid oxidase expression blocks formalin-induced tonic pain. Biochem Biophys Res Commun. 2012; 421:501–507. PMID: 22521889.
Article31. Ge Y, Jiao Y, Li P, Xiang Z, Li Z, Wang L, Li W, Gao H, Shao J, Wen D, Yu W. Coregulation of endoplasmic reticulum stress and oxidative stress in neuropathic pain and disinhibition of the spinal nociceptive circuitry. Pain. 2018; 159:894–906. PMID: 29351168.
Article32. Nieto FR, Cendán CM, Cañizares FJ, Cubero MA, Vela JM, Fernández-Segura E, Baeyens JM. Genetic inactivation and pharmacological blockade of sigma-1 receptors prevent paclitaxel-induced sensory-nerve mitochondrial abnormalities and neuropathic pain in mice. Mol Pain. 2014; 10:11. PMID: 24517272.
Article33. Ruan JP, Zhang HX, Lu XF, Liu YP, Cao JL. EphrinBs/EphBs signaling is involved in modulation of spinal nociceptive processing through a mitogen-activated protein kinases-dependent mechanism. Anesthesiology. 2010; 112:1234–1249. PMID: 20395829.
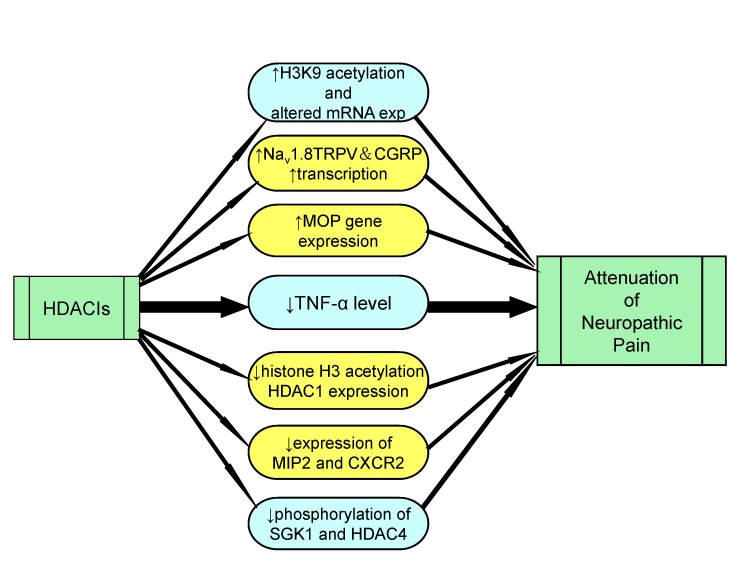
Article34. Khangura RK, Bali A, Jaggi AS, Singh N. Histone acetylation and histone deacetylation in neuropathic pain: an unresolved puzzle? Eur J Pharmacol. 2017; 795:36–42. PMID: 27916557.
Article35. Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011; 333:1462–1466. PMID: 21903816.
Article36. Tan W, Yao WL, Hu R, Lv YY, Wan L, Zhang CH, Zhu C. Alleviating neuropathic pain mechanical allodynia by increasing Cdh1 in the anterior cingulate cortex. Mol Pain. 2015; 11:56. PMID: 26364211.
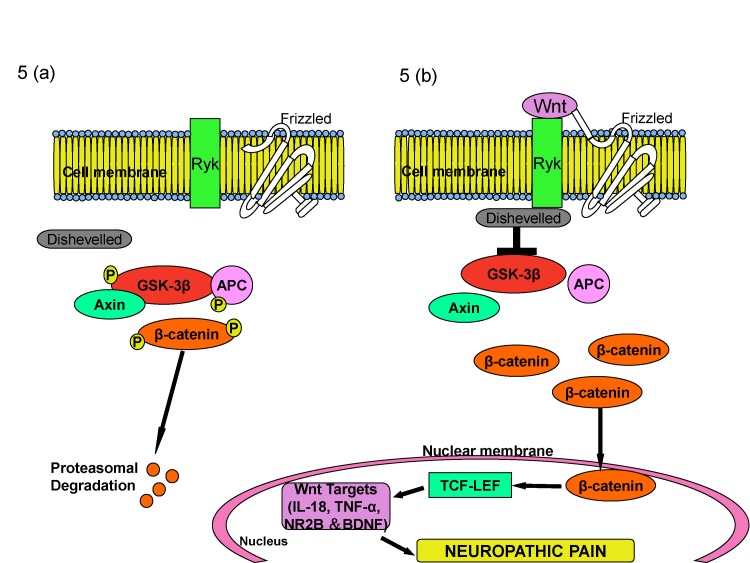
Article37. Zhang X, Chen G, Xue Q, Yu B. Early changes of beta-Catenins and Menins in spinal cord dorsal horn after peripheral nerve injury. Cell Mol Neurobiol. 2010; 30:885–890. PMID: 20369282.38. Liu S, Liu YP, Huang ZJ, Zhang YK, Song AA, Ma PC, Song XJ. Wnt/Ryk signaling contributes to neuropathic pain by regulating sensory neuron excitability and spinal synaptic plasticity in rats. Pain. 2015; 156:2572–2584. PMID: 26407042.
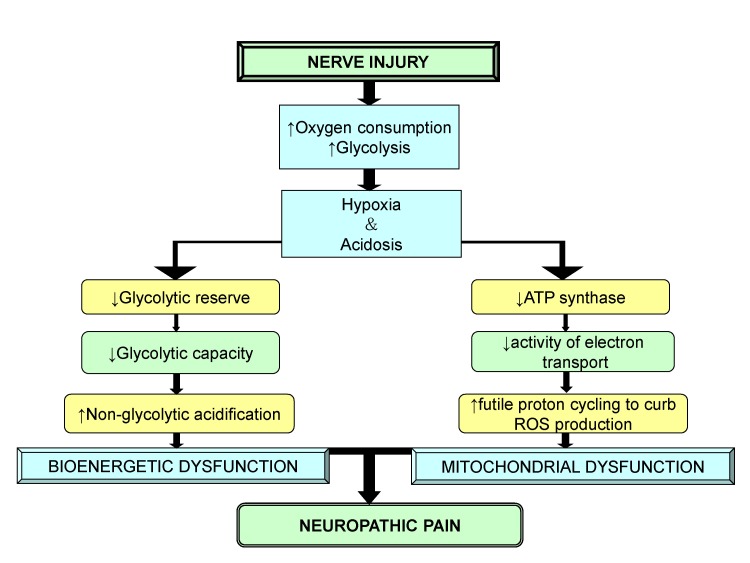
Article39. Lim TK, Rone MB, Lee S, Antel JP, Zhang J. Mitochondrial and bioenergetic dysfunction in trauma-induced painful peripheral neuropathy. Mol Pain. 2015; 11:58. PMID: 26376783.
Article40. Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976; 197:517–532. PMID: 945347.41. Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992; 13:85–86. PMID: 1315463.
Article42. Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007; 131:596–610. PMID: 17981125.43. Tchedre KT, Huang RQ, Dibas A, Krishnamoorthy RR, Dillon GH, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci. 2008; 49:4993–5002. PMID: 18641291.
Article44. Zamanillo D, Romero L, Merlos M, Vela JM. Sigma 1 receptor: a new therapeutic target for pain. Eur J Pharmacol. 2013; 716:78–93. PMID: 23500210.
Article45. Bangaru ML, Weihrauch D, Tang QB, Zoga V, Hogan Q, Wu HE. Sigma-1 receptor expression in sensory neurons and the effect of painful peripheral nerve injury. Mol Pain. 2013; 9:47. PMID: 24015960.
Article46. Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Na HS, Lee JH. Intrathecal injection of the sigma(1) receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology. 2008; 109:879–889. PMID: 18946301.47. de la Puente B, Nadal X, Portillo-Salido E, Sánchez-Arroyos R, Ovalle S, Palacios G, Muro A, Romero L, Entrena JM, Baeyens JM, López-García JA, Maldonado R, Zamanillo D, Vela JM. Sigma-1 receptors regulate activity-induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain. 2009; 145:294–303. PMID: 19505761.48. Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004; 24:8310–8321. PMID: 15385614.
Article49. Xin WJ, Gong QJ, Xu JT, Yang HW, Zang Y, Zhang T, Li YY, Liu XG. Role of phosphorylation of ERK in induction and maintenance of LTP of the C-fiber evoked field potentials in spinal dorsal horn. J Neurosci Res. 2006; 84:934–943. PMID: 16902997.
Article50. Chen Z, Muscoli C, Doyle T, Bryant L, Cuzzocrea S, Mollace V, Mastroianni R, Masini E, Salvemini D. NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain. 2010; 149:100–106. PMID: 20167432.
Article51. Ji RR, Gereau RW 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009; 60:135–148. PMID: 19150373.
Article52. Romero L, Zamanillo D, Nadal X, Sánchez-Arroyos R, Rivera-Arconada I, Dordal A, Montero A, Muro A, Bura A, Segalés C, Laloya M, Hernández E, Portillo-Salido E, Escriche M, Codony X, Encina G, Burgueño J, Merlos M, Baeyens JM, Giraldo J, et al. Pharmacological properties of S1RA, a new sigma-1 receptor antagonist that inhibits neuropathic pain and activity-induced spinal sensitization. Br J Pharmacol. 2012; 166:2289–2306. PMID: 22404321.
Article53. Nieto FR, Cendán CM, Sánchez-Fernández C, Cobos EJ, Entrena JM, Tejada MA, Zamanillo D, Vela JM, Baeyens JM. Role of sigma-1 receptors in paclitaxel-induced neuropathic pain in mice. J Pain. 2012; 13:1107–1121. PMID: 23063344.
Article54. Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol. 2011; 232:154–161. PMID: 21907196.
Article55. Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009; 46:821–831. PMID: 19265700.
Article56. Huang Q, Mao XF, Wu HY, Liu H, Sun ML, Wang X, Wang YX. Cynandione A attenuates neuropathic pain through p38β MAPK-mediated spinal microglial expression of β-endorphin. Brain Behav Immun. 2017; 62:64–77. PMID: 28189715.
Article57. Moon JY, Roh DH, Yoon SY, Kang SY, Choi SR, Kwon SG, Choi HS, Han HJ, Beitz AJ, Lee JH. Sigma-1 receptor-mediated increase in spinal p38 MAPK phosphorylation leads to the induction of mechanical allodynia in mice and neuropathic rats. Exp Neurol. 2013; 247:383–391. PMID: 23333567.
Article58. Lee SA, Park JK, Kang EK, Bae HR, Bae KW, Park HT. Calmodul-independent activation of p38 and p42/44 mitogen-activated protein kinases contributes to c-fos expression by calcium in PC12 cells: modulation by nitric oxide. Brain Res Mol Brain Res. 2000; 75:16–24. PMID: 10648884.
Article59. Roh DH, Choi SR, Yoon SY, Kang SY, Moon JY, Kwon SG, Han HJ, Beitz AJ, Lee JH. Spinal neuronal NOS activation mediates sigma-1 receptor-induced mechanical and thermal hypersensitivity in mice: involvement of PKC-dependent GluN1 phosphorylation. Br J Pharmacol. 2011; 163:1707–1720. PMID: 21391983.
Article60. Martuscello RT, Spengler RN, Bonoiu AC, Davidson BA, Helinski J, Ding H, Mahajan S, Kumar R, Bergey EJ, Knight PR, Prasad PN, Ignatowski TA. Increasing TNF levels solely in the rat hippocampus produces persistent pain-like symptoms. Pain. 2012; 153:1871–1882. PMID: 22770843.
Article61. Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011; 36:979–992. PMID: 21289602.
Article62. Kotagale NR, Shirbhate SH, Shukla P, Ugale RR. Agmatine attenuates neuropathic pain in sciatic nerve ligated rats: modulation by hippocampal sigma receptors. Eur J Pharmacol. 2013; 714:424–431. PMID: 23872381.
Article63. Choi SR, Roh DH, Yoon SY, Kang SY, Moon JY, Kwon SG, Choi HS, Han HJ, Beitz AJ, Oh SB, Lee JH. Spinal sigma-1 receptors activate NADPH oxidase 2 leading to the induction of pain hypersensitivity in mice and mechanical allodynia in neuropathic rats. Pharmacol Res. 2013; 74:56–67. PMID: 23732704.
Article64. Torres LM, Revnic J, Knight AD, Perelman M. Relationship between onset of pain relief and patient satisfaction with fentanyl pectin nasal spray for breakthrough pain in cancer. J Palliat Med. 2014; 17:1150–1157. PMID: 25211772.
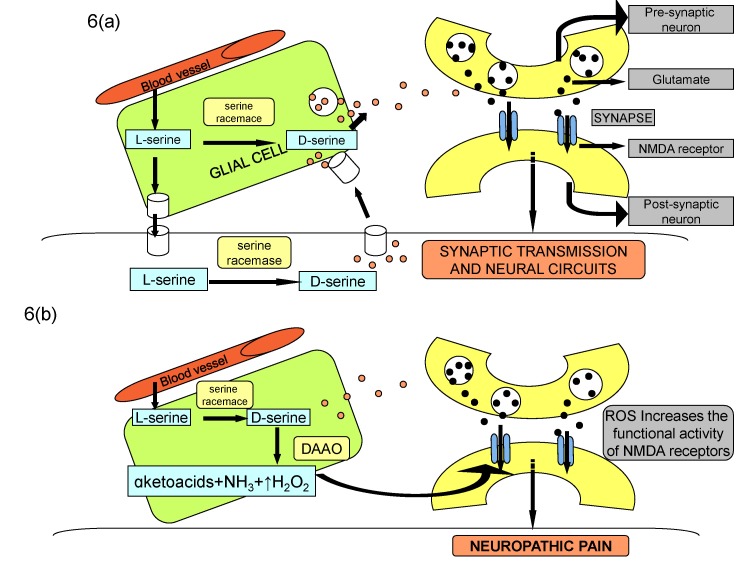
Article65. Moon JY, Choi SR, Roh DH, Yoon SY, Kwon SG, Choi HS, Kang SY, Han HJ, Kim HW, Beitz AJ, Oh SB, Lee JH. Spinal sigma-1 receptor activation increases the production of D-serine in astrocytes which contributes to the development of mechanical allodynia in a mouse model of neuropathic pain. Pharmacol Res. 2015; 100:353–364. PMID: 26316425.
Article66. Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004; 16:580–589. PMID: 15363810.
Article67. Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003; 23:7789–7800. PMID: 12944508.
Article68. Battaglia AA, Sehayek K, Grist J, McMahon SB, Gavazzi I. EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci. 2003; 6:339–340. PMID: 12640461.
Article69. Slack S, Battaglia A, Cibert-Goton V, Gavazzi I. EphrinB2 induces tyrosine phosphorylation of NR2B via Src-family kinases during inflammatory hyperalgesia. Neuroscience. 2008; 156:175–183. PMID: 18694808.
Article70. Kobayashi H, Kitamura T, Sekiguchi M, Homma MK, Kabuyama Y, Konno S, Kikuchi S, Homma Y. Involvement of EphB1 receptor/EphrinB2 ligand in neuropathic pain. Spine (Phila Pa 1976). 2007; 32:1592–1598. PMID: 17621205.
Article71. Song XJ, Cao JL, Li HC, Zheng JH, Song XS, Xiong LZ. Upregulation and redistribution of ephrinB and EphB receptor in dorsal root ganglion and spinal dorsal horn neurons after peripheral nerve injury and dorsal rhizotomy. Eur J Pain. 2008; 12:1031–1039. PMID: 18321739.
Article72. Zhao J, Yuan G, Cendan CM, Nassar MA, Lagerström MC, Kullander K, Gavazzi I, Wood JN. Nociceptor-expressed ephrin-B2 regulates inflammatory and neuropathic pain. Mol Pain. 2010; 6:77. PMID: 21059214.
Article73. Yu LN, Sun LH, Wang M, Wang LJ, Wu Y, Yu J, Wang WN, Zhang FJ, Li X, Yan M. EphrinB-EphB signaling induces hyperalgesia through ERK5/CREB pathway in rats. Pain Physician. 2017; 20:E563–E574. PMID: 28535565.74. Yu LN, Zhou XL, Yu J, Huang H, Jiang LS, Zhang FJ, Cao JL, Yan M. PI3K contributed to modulation of spinal nociceptive information related to ephrinBs/EphBs. PLoS One. 2012; 7:e40930. PMID: 22879882.
Article75. Zhou XL, Zhang CJ, Wang Y, Wang M, Sun LH, Yu LN, Cao JL, Yan M. EphrinB-EphB signaling regulates spinal pain processing via PKCγ. Neuroscience. 2015; 307:64–72. PMID: 26318332.
Article76. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005; 115:2656–2664. PMID: 16200199.
Article77. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002; 110:1389–1398. PMID: 12438434.
Article78. Beggs S, Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol. 2010; 20:474–480. PMID: 20817512.
Article79. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529. PMID: 17565364.
Article80. Gotoh T, Endo M, Oike Y. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int J Inflam. 2011; 2011:259462. PMID: 21755026.
Article81. Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006; 26:3071–3084. PMID: 16581782.82. Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004; 361:184–187. PMID: 15135924.
Article83. Zhan X, Qian B, Cao F, Wu W, Yang L, Guan Q, Gu X2, Okusolubo TA, Dunn SL, Zhu JK, Zhu J. An Arabidopsis PWI and RRM motif-containing protein is critical for pre-mRNA splicing and ABA responses. Nat Commun. 2015; 6:8139. PMID: 26404089.
Article84. Yang F, Whang J, Derry WT, Vardeh D, Scholz J. Analgesic treatment with pregabalin does not prevent persistent pain after peripheral nerve injury in the rat. Pain. 2014; 155:356–366. PMID: 24176928.
Article85. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002; 415:92–96. PMID: 11780124.
Article86. Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003; 4:321–329. PMID: 12612580.
Article87. Molinari M, Galli C, Piccaluga V, Pieren M, Paganetti P. Sequential assistance of molecular chaperones and transient formation of covalent complexes during protein degradation from the ER. J Cell Biol. 2002; 158:247–257. PMID: 12119363.
Article88. Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci USA. 2015; 112:9082–9087. PMID: 26150506.
Article89. Kwiatkowski AV, Weis WI, Nelson WJ. Catenins: playing both sides of the synapse. Curr Opin Cell Biol. 2007; 19:551–556. PMID: 17936606.
Article90. Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol. 2012; 4:a008003. PMID: 22300976.
Article91. He X. Wnt signaling went derailed again: a new track via the LIN-18 receptor? Cell. 2004; 118:668–670. PMID: 15369666.92. Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999; 398:431–436. PMID: 10201374.93. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997; 276:404–407. PMID: 9103196.
Article94. Sawicki M, Chen G, Farley S. Menin Is a beta-catenin associated agonist of the wnt signaling pathway in pancreatic endocrine cells. J Surg Res. 2008; 144:196.95. Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013; 123:2268–2286. PMID: 23585476.
Article96. Itokazu T, Hayano Y, Takahashi R, Yamashita T. Involvement of Wnt/β-catenin signaling in the development of neuropathic pain. Neurosci Res. 2014; 79:34–40. PMID: 24361264.
Article97. Yang QO, Yang WJ, Li J, Liu FT, Yuan H, Ou Yang YP. Ryk receptors on unmyelinated nerve fibers mediate excitatory synaptic transmission and CCL2 release during neuropathic pain induced by peripheral nerve injury. Mol Pain. 2017; 13:1744806917709372. PMID: 28565999.
Article98. Gao K, Wang YS, Yuan YJ, Wan ZH, Yao TC, Li HH, Tang PF, Mei XF. Neuroprotective effect of rapamycin on spinal cord injury via activation of the Wnt/β-catenin signaling pathway. Neural Regen Res. 2015; 10:951–957. PMID: 26199613.
Article99. Xu Z, Chen Y, Yu J, Yin D, Liu C, Chen X, Zhang D. TCF4 mediates the maintenance of neuropathic pain through Wnt/β-catenin signaling following peripheral nerve injury in rats. J Mol Neurosci. 2015; 56:397–408. PMID: 25963533.
Article100. Lüthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998; 21:9–12. PMID: 9697847.101. Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004; 471:241–276. PMID: 14991560.
Article102. George MS, Abbott LF, Siegelbaum SA. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K+ channels. Nat Neurosci. 2009; 12:577–584. PMID: 19363490.103. Liu X, Zhang L, Jin L, Tan Y, Li W, Tang J. HCN2 contributes to oxaliplatin-induced neuropathic pain through activation of the CaMKII/CREB cascade in spinal neurons. Mol Pain. 2018; 14:1744806918778490. PMID: 29806529.
Article104. Yeon KY, Chung G, Kim YH, Hwang JH, Davies AJ, Park MK, Ahn DK, Kim JS, Jung SJ, Oh SB. Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain. 2011; 152:2108–2116. PMID: 21664051.
Article105. Du L, Wang SJ, Cui J, He WJ, Ruan HZ. Inhibition of HCN channels within the periaqueductal gray attenuates neuropathic pain in rats. Behav Neurosci. 2013; 127:325–329. PMID: 23398435.
Article106. Young GT, Emery EC, Mooney ER, Tsantoulas C, McNaughton PA. Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain. 2014; 155:1708–1719. PMID: 24861581.
Article107. Smith T, Al Otaibi M, Sathish J, Djouhri L. Increased expression of HCN2 channel protein in L4 dorsal root ganglion neurons following axotomy of L5- and inflammation of L4-spinal nerves in rats. Neuroscience. 2015; 295:90–102. PMID: 25813712.
Article108. Cordeiro Matos S, Zhang Z, Séguéla P. Peripheral neuropathy induces HCN channel dysfunction in pyramidal neurons of the medial prefrontal cortex. J Neurosci. 2015; 35:13244–13256. PMID: 26400952.
Article109. Angermüller S, Islinger M, Völkl A. Peroxisomes and reactive oxygen species, a lasting challenge. Histochem Cell Biol. 2009; 131:459–463. PMID: 19224237.
Article110. Wang YX, Gong N, Xin YF, Hao B, Zhou XJ, Pang CC. Biological implications of oxidation and unidirectional chiral inversion of D-amino acids. Curr Drug Metab. 2012; 13:321–331. PMID: 22304623.
Article111. Yoshikawa M, Oka T, Kawaguchi M, Hashimoto A. MK-801 up-regulates the expression of d-amino acid oxidase mRNA in rat brain. Brain Res Mol Brain Res. 2004; 131:141–144. PMID: 15530664.
Article112. Lu JM, Gong N, Wang YC, Wang YX. D-Amino acid oxidase-mediated increase in spinal hydrogen peroxide is mainly responsible for formalin-induced tonic pain. Br J Pharmacol. 2012; 165:1941–1955. PMID: 21950354.
Article113. Williams M. Commentary: genome-based CNS drug discovery: D-amino acid oxidase (DAAO) as a novel target for antipsychotic medications: progress and challenges. Biochem Pharmacol. 2009; 78:1360–1365. PMID: 19591808.
Article114. Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007; 76:75–100. PMID: 17362198.
Article115. Lin TB, Hsieh MC, Lai CY, Cheng JK, Chau YP, Ruan T, Chen GD, Peng HY. Modulation of nerve injury-induced HDAC4 cytoplasmic retention contributes to neuropathic pain in rats. Anesthesiology. 2015; 123:199–212. PMID: 25871743.
Article116. Cherng CH, Lee KC, Chien CC, Chou KY, Cheng YC, Hsin ST, Lee SO, Shen CH, Tsai RY, Wong CS. Baicalin ameliorates neuropathic pain by suppressing HDAC1 expression in the spinal cord of spinal nerve ligation rats. J Formos Med Assoc. 2014; 113:513–520. PMID: 23684218.
Article117. Kukkar A, Singh N, Jaggi AS. Attenuation of neuropathic pain by sodium butyrate in an experimental model of chronic constriction injury in rats. J Formos Med Assoc. 2014; 113:921–928. PMID: 23870713.
Article118. Hobo S, Eisenach JC, Hayashida K. Up-regulation of spinal glutamate transporters contributes to anti-hypersensitive effects of valproate in rats after peripheral nerve injury. Neurosci Lett. 2011; 502:52–55. PMID: 21802494.
Article119. Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RW 4th, Copani A, Nicoletti F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009; 75:1014–1020. PMID: 19255242.
Article120. Zammataro M, Sortino MA, Parenti C, Gereau RW 4th, Chiechio S. HDAC and HAT inhibitors differently affect analgesia mediated by group II metabotropic glutamate receptors. Mol Pain. 2014; 10:68. PMID: 25406541.
Article121. Kami K, Taguchi S, Tajima F, Senba E. Histone acetylation in microglia contributes to exercise-induced hypoalgesia in neuropathic pain model mice. J Pain. 2016; 17:588–599. PMID: 26844418.
Article122. Uchida H, Matsushita Y, Araki K, Mukae T, Ueda H. Histone deacetylase inhibitors relieve morphine resistance in neuropathic pain after peripheral nerve injury. J Pharmacol Sci. 2015; 128:208–211. PMID: 26318673.
Article123. Zhang Y, Gong K, Zhou W, Shao G, Li S, Lin Q, Li J. Involvement of subtypesγ and ε of protein kinase C in colon pain induced by formalin injection. Neurosignals. 2011; 19:142–150. PMID: 21701146.124. Sas K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007; 257:221–239. PMID: 17462670.
Article125. Janes K, Doyle T, Bryant L, Esposito E, Cuzzocrea S, Ryerse J, Bennett GJ, Salvemini D. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain. 2013; 154:2432–2440. PMID: 23891899.
Article126. Chen KH, Lin CR, Cheng JT, Cheng JK, Liao WT, Yang CH. Altered mitochondrial ATP synthase expression in the rat dorsal root ganglion after sciatic nerve injury and analgesic effects of intrathecal ATP. Cell Mol Neurobiol. 2014; 34:51–59. PMID: 24048632.
Article127. Inoue K. Neuropharmacological study of ATP receptors, especially in the relationship between glia and pain. Yakugaku Zasshi. 2017; 137:563–569. PMID: 28458288.
Article128. Steen KH, Steen AE, Kreysel HW, Reeh PW. Inflammatory mediators potentiate pain induced by experimental tissue acidosis. Pain. 1996; 66:163–170. PMID: 8880837.
Article129. Wong AS, Gumbiner BM. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol. 2003; 161:1191–1203. PMID: 12810698.
Article130. Husseman JW, Nochlin D, Vincent I. Mitotic activation: a convergent mechanism for a cohort of neurodegenerative diseases. Neurobiol Aging. 2000; 21:815–828. PMID: 11124425.
Article131. Puram SV, Bonni A. Novel functions for the anaphase-promoting complex in neurobiology. Semin Cell Dev Biol. 2011; 22:586–594. PMID: 21439392.
Article132. Fu AK, Hung KW, Fu WY, Shen C, Chen Y, Xia J, Lai KO, Ip NY. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci. 2011; 14:181–189. PMID: 21186356.
Article133. Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011; 152:1641–1648. PMID: 21474245.
Article134. Tan Y, Wang J, Su S, Wang Q, Jiang S, Lu L, Chen YH. Enhancement of endocytic uptake of HIV-1 virions into CD4-negative epithelial cells by HIV-1 gp41 via its interaction with POB1. Cell Mol Immunol. 2017; 14:568–571. PMID: 28579616.
Article135. Zhao WJ, Gao ZY, Wei H, Nie HZ, Zhao Q, Zhou XJ, Wang YX. Spinal D-amino acid oxidase contributes to neuropathic pain in rats. J Pharmacol Exp Ther. 2010; 332:248–254. PMID: 19828879.
Article136. Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical toxicities of histone deacetylase inhibitors. Pharmaceuticals (Basel). 2010; 3:2751–2767. PMID: 27713375.
Article