Korean J Physiol Pharmacol.
2019 May;23(3):219-227. 10.4196/kjpp.2019.23.3.219.
The role of calmodulin in regulating calcium-permeable PKD2L1 channel activity
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. insuk@snu.ac.kr
- KMID: 2443609
- DOI: http://doi.org/10.4196/kjpp.2019.23.3.219
Abstract
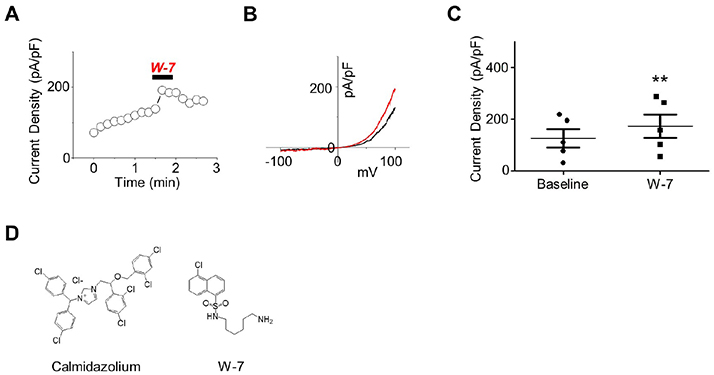
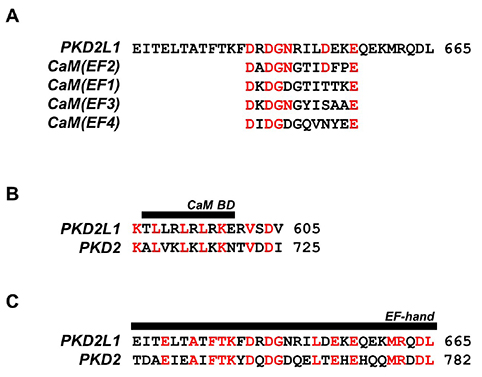
- Polycystic kidney disease 2-like-1 (PKD2L1), polycystin-L or transient receptor potential polycystin 3 (TRPP3) is a TRP superfamily member. It is a calcium-permeable non-selective cation channel that regulates intracellular calcium concentration and thereby calcium signaling. Although the calmodulin (CaM) inhibitor, calmidazolium, is an activator of the PKD2L1 channel, the activating mechanism remains unclear. The purpose of this study is to clarify whether CaM takes part in the regulation of the PKD2L1 channel, and if so, how. With patch clamp techniques, we observed the current amplitudes of PKD2L1 significantly reduced when coexpressed with CaM and CaMΔN. This result suggests that the N-lobe of CaM carries a more crucial role in regulating PKD2L1 and guides us into our next question on the different functions of two lobes of CaM. We also identified the predicted CaM binding site, and generated deletion and truncation mutants. The mutants showed significant reduction in currents losing PKD2L1 current-voltage curve, suggesting that the C-terminal region from 590 to 600 is crucial for maintaining the functionality of the PKD2L1 channel. With PKD2L1608Stop mutant showing increased current amplitudes, we further examined the functional importance of EF-hand domain. Along with co-expression of CaM, ΔEF-hand mutant also showed significant changes in current amplitudes and potentiation time. Our findings suggest that there is a constitutive inhibition of EF-hand and binding of CaM C-lobe on the channel in low calcium concentration. At higher calcium concentration, calcium ions occupy the N-lobe as well as the EF-hand domain, allowing the two to compete to bind to the channel.
MeSH Terms
Figure
Cited by 1 articles
-
Ca2+/calmodulin-dependent regulation of polycystic kidney disease 2-like-1 by binding at C-terminal domain
Julia Young Baik, Eunice Yon June Park, Insuk So
Korean J Physiol Pharmacol. 2020;24(3):277-286. doi: 10.4196/kjpp.2020.24.3.277.
Reference
-
1. DeCaen PG, Liu X, Abiria S, Clapham DE. Atypical calcium regulation of the PKD2-L1 polycystin ion channel. Elife. 2016; 5:e13413.
Article2. Chen XZ, Vassilev PM, Basora N, Peng JB, Nomura H, Segal Y, Brown EM, Reeders ST, Hediger MA, Zhou J. Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature. 1999; 401:383–386.
Article3. Park EYJ, Kwak M, Ha K, So I. Identification of clustered phosphorylation sites in PKD2L1: how PKD2L1 channel activation is regulated by cyclic adenosine monophosphate signaling pathway. Pflugers Arch. 2018; 470:505–516.
Article4. Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013; 504:311–314.
Article5. Yao G, Luo C, Harvey M, Wu M, Schreiber TH, Du Y, Basora N, Su X, Contreras D, Zhou J. Disruption of polycystin-L causes hippocampal and thalamocortical hyperexcitability. Hum Mol Genet. 2016; 25:448–458.
Article6. Sternberg JR, Prendergast AE, Brosse L, Cantaut-Belarif Y, Thouvenin O, Orts-Del'Immagine A, Castillo L, Djenoune L, Kurisu S, McDearmid JR, Bardet PL, Boccara C, Okamoto H, Delmas P, Wyart C. Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat Commun. 2018; 9:3804.
Article7. Su Q, Hu F, Liu Y, Ge X, Mei C, Yu S, Shen A, Zhou Q, Yan C, Lei J, Zhang Y, Liu X, Wang T. Cryo-EM structure of the polycystic kidney disease-like channel PKD2L1. Nat Commun. 2018; 9:1192.
Article8. Hulse RE, Li Z, Huang RK, Zhang J, Clapham DE. Cryo-EM structure of the polycystin 2-l1 ion channel. Elife. 2018; 7:e36931.
Article9. Saimi Y, Kung C. Calmodulin as an ion channel subunit. Annu Rev Physiol. 2002; 64:289–311.
Article10. Zhu MX. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005; 451:105–115.11. Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997; 11:331–340.
Article12. Sunagawa M, Kosugi T, Nakamura M, Sperelakis N. Pharmacological actions of calmidazolium, a calmodulin antagonist, in cardiovascular system. Cardiovasc Drug Rev. 2000; 18:211–221.
Article13. DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013; 504:315–318.
Article14. Singh AK, McGoldrick LL, Twomey EC, Sobolevsky AI. Mechanism of calmodulin inactivation of the calcium-selective TRP channel TRPV6. Sci Adv. 2018; 4:eaau6088.
Article15. Lee CH, MacKinnon R. Activation mechanism of a human SK-calmodulin channel complex elucidated by cryo-EM structures. Science. 2018; 360:508–513.
Article16. Wang C, Chung BC, Yan H, Wang HG, Lee SY, Pitt GS. Structural analyses of Ca2+/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nat Commun. 2014; 5:4896.
Article17. Osawa M, Swindells MB, Tanikawa J, Tanaka T, Mase T, Furuya T, Ikura M. Solution structure of calmodulin-W-7 complex: the basis of diversity in molecular recognition. J Mol Biol. 1998; 276:165–176.
Article18. Hughes TET, Pumroy RA, Yazici AT, Kasimova MA, Fluck EC, Huynh KW, Samanta A, Molugu SK, Zhou ZH, Carnevale V, Rohacs T, Moiseenkova-Bell VY. Structural insights on TRPV5 gating by endogenous modulators. Nat Commun. 2018; 9:4198.
Article19. Hasan R, Zhang X. Ca2+ Regulation of TRP ion channels. Int J Mol Sci. 2018; 19:1256.20. Lau SY, Procko E, Gaudet R. Distinct properties of Ca2+-calmodulin binding to N- and C-terminal regulatory regions of the TRPV1 channel. J Gen Physiol. 2012; 140:541–555.21. Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007; 54:905–918.
Article22. Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, Janowsky A, Fakler B, Adelman JP, Maylie J. Domains responsible for constitutive and Ca2+-dependent interactions between calmodulin and small conductance Ca2+-activated potassium channels. J Neurosci. 1999; 19:8830–8838.23. Shah VN, Wingo TL, Weiss KL, Williams CK, Balser JR, Chazin WJ. Calcium-dependent regulation of the voltage-gated sodium channel hH1: intrinsic and extrinsic sensors use a common molecular switch. Proc Natl Acad Sci U S A. 2006; 103:3592–3597.
Article24. Shah VN, Chagot B, Chazin WJ. Calcium-dependent regulation of ion channels. Calcium Bind Proteins. 2006; 1:203–212.25. Zheng W, Hussein S, Yang J, Huang J, Zhang F, Hernandez-Anzaldo S, Fernandez-Patron C, Cao Y, Zeng H, Tang J, Chen XZ. A novel PKD2L1 C-terminal domain critical for trimerization and channel function. Sci Rep. 2015; 5:9460.
Article26. Shen PS, Yang X, DeCaen PG, Liu X, Bulkley D, Clapham DE, Cao E. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell. 2016; 167:763–773.e11.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ca2+/calmodulin-dependent regulation of polycystic kidney disease 2-like-1 by binding at C-terminal domain
- Role of Calmodulin in the Generation of Reactive Oxygen Species and Apoptosis Induced by Tamoxifen in HepG2 Human Hepatoma Cells
- EF-hand like Region in the N-terminus of Anoctamin 1 Modulates Channel Activity by Ca²⺠and Voltage
- Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1
- The Effect of Verapamil on the Specific Activity of Na+-K+-activated Adenosine Triphosphatase in Rabbit Renal Medulla