Dement Neurocogn Disord.
2015 Dec;14(4):149-157. 10.12779/dnd.2015.14.4.149.
Effect of Illiteracy on Cognition and Cerebral Morphology in Later Life
- Affiliations
-
- 1Department of Neurology, School of Medicine, Catholic University of Daegu, Daegu, Korea. dolbaeke@cu.ac.kr
- 2Department of Biomedical Engineering, Catholic University of Daegu, Daegu, Korea.
- 3Department of Neurology, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2442932
- DOI: http://doi.org/10.12779/dnd.2015.14.4.149
Abstract
- BACKGROUND AND PURPOSE
A better developmental environment has positive effects on brain development. The acquisition of literacy during childhood may affect brain functional organization. The present study aimed to evaluate the effects of illiteracy on neuropsychological test results and cerebral morphology in later life.
METHODS
We recruited 7 illiterate elderly farmers who had never attended school and had no reading or writing knowledge. These subjects were compared with 9 literate subjects in terms of neuropsychological performance and brain volume. All subjects were over 65-years-old and had the same regional and occupational background.
RESULTS
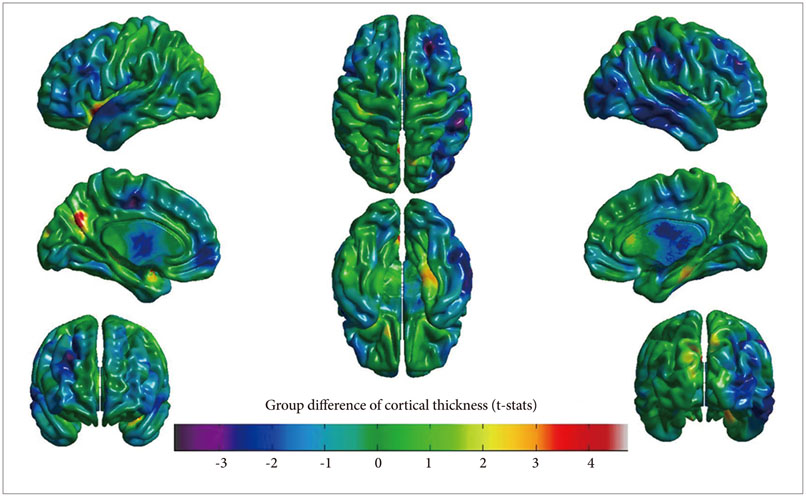
Neuropsychological tests indicated that the performance of the illiterate subjects was worse than that of literate subjects in all cognitive domains except forward digit span, tool-use and tool-free gestures, verbal word recognition, and verbal generation of animals and grocery items. The illiterate group also showed significantly decreased cortical volume and surface area in both parietal lobes. However, the illiterate group showed increased cortical thickness in the left cuneus.
CONCLUSIONS
Literacy acquired in childhood may increase the volume of the parietal lobe and improve neuropsychological performance through the process of brain plasticity. The effects can be lifelong.
MeSH Terms
Figure
Reference
-
1. Bornstein MH. On the development of color naming in young children: data and theory. Brain Lang. 1985; 26:72–93.
Article2. Krech D, Rosenzweig MR, Bennett EL. Effects of environmental complexity and training on brain chemistry. J Comp Physiol Psychol. 1960; 53:509–519.
Article3. Diamond MC. Enriching heredity: The impact of the environment on the anatomy of the brain. New York: The Free Press;1988.4. Beaulieu C, Cynader M. Effect of the richness of the environment on neurons in cat visual cortex. I Receptive field properties. Brain Res Dev Brain Res. 1990; 53:71–78.
Article5. Beaulieu C, Colonnier M. Number and size of neurons and synapses in the motor cortex of cats raised in different environmental complexities. J Comp Neurol. 1989; 289:178–181.
Article6. Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988; 23:138–144.
Article7. Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993; 43:13–20.
Article8. Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003; 60:1909–1915.
Article9. Brayne C, Calloway P. The association of education and socioeconomic status with the Mini Mental State Examination and the clinical diagnosis of dementia in elderly people. Age Ageing. 1990; 19:91–96.
Article10. Zhou DF, Wu CS, Qi H, Fan JH, Sun XD, Como P, et al. Prevalence of dementia in rural China: impact of age, gender and education. Acta Neurol Scand. 2006; 114:273–280.
Article11. Schmand B, Smit J, Lindeboom J, Smits C, Hooijer C, Jonker C, et al. Low education is a genuine risk factor for accelerated memory decline and dementia. J Clin Epidemiol. 1997; 50:1025–1033.
Article12. Eisenberg DP, London ED, Matochik JA, Derbyshire S, Cohen LJ, Steinfeld M, et al. Education-associated cortical glucose metabolism during sustained attention. Neuroreport. 2005; 16:1473–1476.
Article13. Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999; 53:189–196.
Article14. Kwon OD, Cho SS, Seo SW, Na DL. Effect of illiteracy on neuropsychological tests and glucose metabolism of brain in later life. J Neuroimaging. 2012; 22:292–298.
Article15. Kang Y, Na DL. Seoul neuropsychological screening battery. Incheon: Human Brain Research & Consulting Co.;2003.16. Ku HM, Kim JH, Gwon UJ, Kim SH, Lee HS, Go HJ, et al. A study on the reliability and validity of Seoul-instrumental activities of daily living (S-IADL). J Korean Neuropsychiatr Assoc. 2004; 43:189–199.17. Evans AC. Brain Development Cooperative Group. The NIH MRI study of normal brain development. Neuroimage. 2006; 30:184–202.
Article18. Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994; 18:192–205.
Article19. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998; 17:87–97.
Article20. Zijdenbos AP, Forghani R, Evans AC. Automatic "pipeline" analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002; 21:1280–1291.
Article21. Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005; 27:210–221.
Article22. Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007; 34:1535–1544.
Article23. Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008; 18:2181–2191.
Article24. Yoon U, Lee JM, Im K, Shin YW, Cho BH, Kim IY, et al. Pattern classification using principal components of cortical thickness and its discriminative pattern in schizophrenia. Neuroimage. 2007; 34:1405–1415.
Article25. Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006; 17:1103–1106.
Article26. Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage. 2006; 29:1224–1230.
Article27. Wiegand LC, Warfield SK, Levitt JJ, Hirayasu Y, Salisbury DF, Heckers S, et al. An in vivo MRI study of prefrontal cortical complexity in first-episode psychosis. Am J Psychiatry. 2005; 162:65–70.
Article28. Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005; 28:635–662.
Article29. Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005; 24:163–173.
Article30. Im K, Lee JM, Seo SW, Yoon U, Kim ST, Kim YH, et al. Variations in cortical thickness with dementia severity in Alzheimer's disease. Neurosci Lett. 2008; 436:227–231.
Article31. Van Essen DC, Drury HA. Structural and functional analyses of human cerebral cortex using a surface-based atlas. J Neurosci. 1997; 17:7079–7102.
Article32. Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 1988; 179:173–179.
Article33. Zilles K, Schleicher A, Rath M, Bauer A. Quantitative receptor autoradiography in the human brain. Methodical aspects. Histochemistry. 1988; 90:129–137.
Article34. Yoon U, Fonov VS, Perusse D, Evans AC. Brain Development Cooperative Group. The effect of template choice on morphometric analysis of pediatric brain data. Neuroimage. 2009; 45:769–777.
Article35. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002; 15:870–878.
Article36. Aghababian V, Nazir TA. Developing normal reading skills: aspects of the visual processes underlying word recognition. J Exp Child Psychol. 2000; 76:123–150.
Article37. Hornberger M, Piguet O, Kipps C, Hodges JR. Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology. 2008; 71:1481–1488.
Article38. Foundas AL, Macauley BL, Raymer AM, Maher LM, Rothi LJ, Heilman KM. Ideomotor apraxia in Alzheimer disease and left hemisphere stroke: limb transitive and intransitive movements. Neuropsychiatry Neuropsychol Behav Neurol. 1999; 12:161–166.39. Poizner H, Mack L, Verfaellie M, Rothi LJ, Heilman KM. Three-dimensional computergraphic analysis of apraxia. Neural representations of learned movement. Brain. 1990; 113(Pt 1):85–101.
Article40. Meador KJ, Loring DW, Lee K, Hughes M, Lee G, Nichols M, et al. Cerebral lateralization: relationship of language and ideomotor praxis. Neurology. 1999; 53:2028–2031.
Article41. Heilman KM. Apraxia. Continuum (Minneap Minn). 2010; 16(4 Behavioral Neurology):86–98.
Article42. Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia. 2000; 38:734–747.
Article43. Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol. 1987; 9:479–497.
Article44. Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;2001.45. Margolin DI, Pate DS, Friedrich FJ, Elia E. Dysnomia in dementia and in stroke patients: different underlying cognitive deficits. J Clin Exp Neuropsychol. 1990; 12:597–612.
Article46. Hart S, Smith CM, Swash M. Word fluency in patients with early dementia of Alzheimer type. Br J Clin Psychol. 1988; 27(Pt 2):115–124.
Article47. Sherman AM, Massman PJ. Prevalence and correlates of category versus letter fluency discrepancies in Alzheimer's disease. Arch Clin Neuropsychol. 1999; 14:411–418.
Article48. Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability). Biol Psychiatry. 2005; 57:1301–1309.
Article49. Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006; 26:10700–10708.
Article50. Kawamura M, Midorikawa A, Kezuka M. Cerebral localization of the center for reading and writing music. Neuroreport. 2000; 11:3299–3303.
Article51. Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, et al. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001; 57:47–54.
Article52. Otsuki M, Soma Y, Arai T, Otsuka A, Tsuji S. Pure apraxic agraphia with abnormal writing stroke sequences: report of a Japanese patient with a left superior parietal haemorrhage. J Neurol Neurosurg Psychiatry. 1999; 66:233–237.
Article53. Nakamura K, Honda M, Okada T, Hanakawa T, Toma K, Fukuyama H, et al. Participation of the left posterior inferior temporal cortex in writing and mental recall of kanji orthography: A functional MRI study. Brain. 2000; 123(Pt 5):954–967.
Article54. Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Hum Brain Mapp. 2009; 30:810–820.
Article55. Gerstmann J. Some notes on the Gerstmann syndrome. Neurology. 1957; 7:866–869.
Article56. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001; 14(1 Pt 1):21–36.
Article57. Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol. 2002; 87:589–607.
Article58. Hurwitz M, Valadao D, Danckert J. Functional MRI of dynamic judgments of spatial extent. Exp Brain Res. 2011; 214:61–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cerebral Aneurysm Arising from the Azygous Anterior Cerebral Artery : Case Report
- Effects of Irrational Parenthood Cognition, Post Traumatic Stress Disorder and Spousal Support on Quality of Life of Infertile Women
- Neurodevelopmental Outcome of Preterm Infants at Childhood: Cognition and Language
- A Study on the Application of a Dementia Prevention Program and It's Effect Test
- The Relationships of Pain cognition, Performance Status, and Hope with Health-related Quality of Life in Cancer Patients