Prog Med Phys.
2019 Mar;30(1):22-31. 10.14316/pmp.2019.30.1.22.
b0 Dependent Neuronal Activation in the Diffusion-Based Functional MRI
- Affiliations
-
- 1Department of Biomedical Engineering, Graduate School, Kyung Hee University, Yongin, Korea.
- 2Department of Radiology, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea. ghjahng@gmail.com
- KMID: 2442418
- DOI: http://doi.org/10.14316/pmp.2019.30.1.22
Abstract
- PURPOSE
To develop a new diffusion-based functional MRI (fMRI) sequence to generate apparent diffusion coefficient (ADC) maps in single excitation and evaluate the contribution of b0 signal on neuronal changes.
MATERIALS AND METHODS
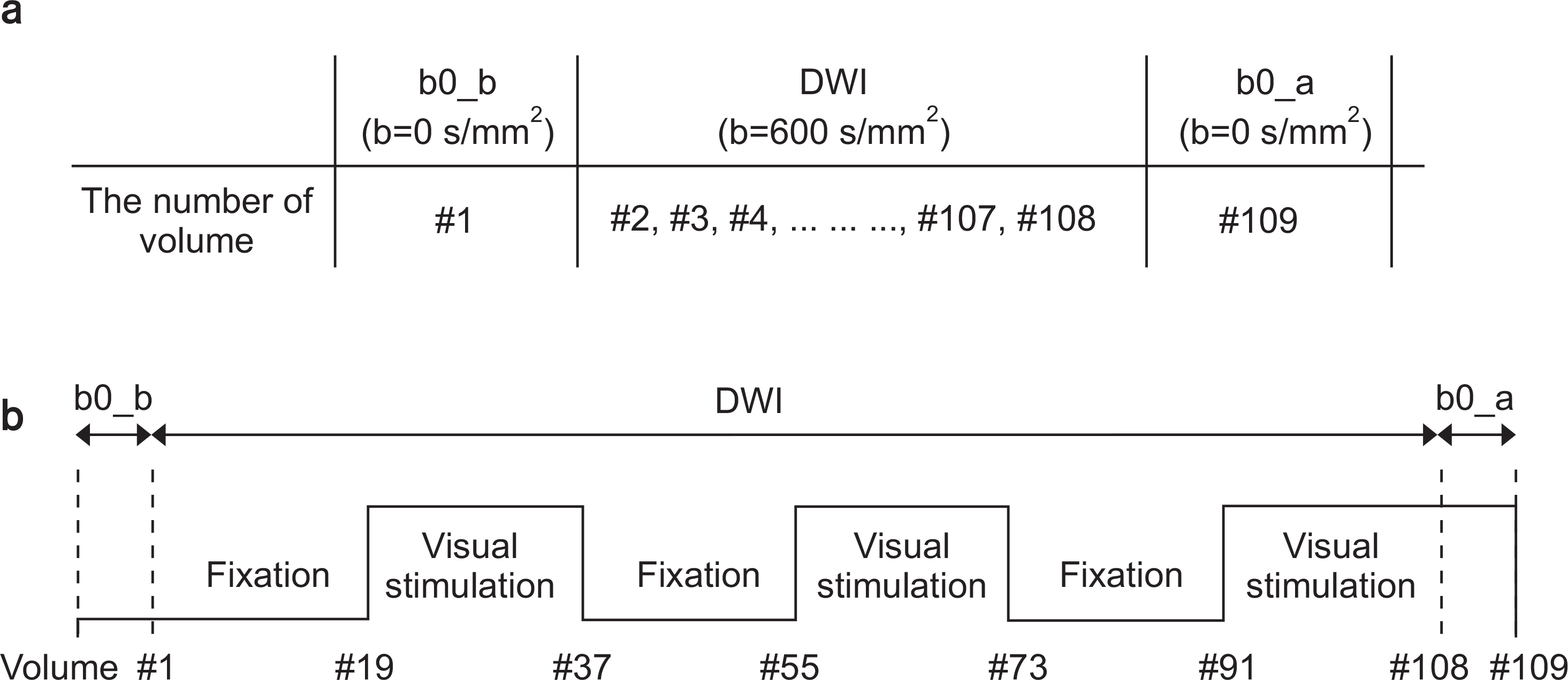
A diffusion-based fMRI sequence was d esigned with single measurement that can acquire images of three directions at a time, obtaining b = 0 s/mm2 during the first baseline condition (b0_b), followed by 107 diffusion-weighted imaging (DWI) with b = 600 s/mm2 during the b aseline and visual stimulation conditions, and another b = 0 s/mm2 during the last activation condition (b0_a). ADC was mapped in three different ways: 1) using b0_b (ADC_b) for all time points, 2) using b0_a (ADC_a) for all time points, and 3) using b0_b and b0_a (ADC_ba) for baseline and stimulation scans, respectively. The fMRI studies were conducted on the brains of 16 young healthy volunteers using visual stimulations in a 3T MRI system. In addition, the blood oxygen level dependent (BOLD) fMRI was also acquired to compare it with diffusion-based fMRI. A sample t-test was used to investigate the voxel-wise average between the subjects.
RESULTS
The BOLD data consisted of only activated voxels. However, ADC_ba data was observed in both deactivated and activated voxels. There were no statistically significant activated or deactivated voxels for DWI, ADC_b, and ADC_a.
CONCLUSIONS
With the new sequence, neuronal activations can be mapped with visual stimulation as compared to the baseline condition in several areas in the brain. We showed that ADC should be mapped using both DWI and b0 images acquired with the same conditions.
Keyword
MeSH Terms
Figure
Reference
-
1.Darquie A., Poline JB., Poupon C., Saint-Jalmes H., Le Bihan D. Transient decrease in water diffusion observed in human occipital cortex during visual stimulation [published online ahead of print 2001/07/19]. Proc Natl Acad Sci U S A. 2001. 98(16):9391–9395.2.Roy CS., Sherrington CS. On the Regulation of the Blood-supply of the Brain [published online ahead of print 1890/01/01]. J Physiol. 1890. 11(1-2):85–158.3.Bandettini PA., Wong EC., Hinks RS., Tikofsky RS., Hyde JS. Time course EPI of human brain function during task activation [published online ahead of print 1992/06/01]. Magn Reson Med. 1992. 25(2):390–397.4.Kwong KK., Belliveau JW., Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation [published online ahead of print 1992/06/15]. Proc Natl Acad Sci U S A. 1992. 89(12):5675–5679.5.Ogawa S., Tank DW., Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging [published online ahead of print 1992/07/01]. Proc Natl Acad Sci U S A. 1992. 89(13):5951–5955.6.Le Bihan D. Molecular diffusion, tissue microdynam-ics and microstructure [published online ahead of print 1995/11/01]. NMR Biomed. 1995. 8(7-8):375–386.7.Latour LL., Svoboda K., Mitra PP., Sotak CH. Time-dependent diffusion of water in a biological model system [published online ahead of print 1994/02/15]. Proc Natl Acad Sci U S A. 1994. 91(4):1229–1233.8.Bae MS., Jahng GH., Ryu CW., Kim EJ., Choi WS., Yang DM. Effect of intravenous gadolinium-DTPA on diffusion tensor MR imaging for the evaluation of brain tumors [published online ahead of print 2009/07/29]. Neuroradiology. 2009. 51(12):793–802.9.Jahng GH., Xu S. Local susceptibility causes diffusion alterations in patients with Alzheimer's disease and mild cognitive impairment [published online ahead of print 2012/03/15]. Brain Imaging Behav. 2012. 6(3):426–436.10.Le Bihan D., Urayama S., Aso T., Hanakawa T., Fukuyama H. Direct and fast detection of neuronal activation in the human brain with diffusion MRI [published online ahead of print 2006/05/17]. Proc Natl Acad Sci U S A. 2006. 103(21):8263–8268.11.Song AW., Guo H., Truong TK. Single-shot ADC imaging for fMRI [published online ahead of print 2007/01/30]. Magn Reson Med. 2007. 57(2):417–422.12.Stejskal EO., Tanner JE. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J Chem Phys. 1964. 42(1):
Article13.Lancaster JL., Tordesillas-Gutierrez D., Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template [published online ahead of print 2007/02/03]. Hum Brain Mapp. 2007. 28(11):1194–1205.14.Friston K., Holmes AP., Worsley KJ., Poline JB., Frith CD., Frackowiak RS. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995. 2:189–210.
Article15.Talairach J., Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme. 1988.16.Nicolas R., Gros-Dagnac H., Aubry F., Celsis P. Comparison of BOLD, diffusion-weighted fMRI and ADC-fMRI for stimulation of the primary visual system with a block paradigm. Magn Reson Imaging. 2017. 39:123–131.
Article17.Williams RJ., Reutens DC., Hocking J. Influence of BOLD Contributions to Diffusion fMRI Activation of the Visual Cortex. Frontiers in neuroscience. 2016. 10:279.
Article18.Zhong J., Kennan RP., Fulbright RK., Gore JC. Quantification of intravascular and extravascular contributions to BOLD effects induced by alteration in oxygenation or intravascular contrast agents [published online ahead of print 1998/10/15]. Magn Reson Med. 1998. 40(4):526–536.19.Jahng GH., Weiner MW., Schuff N. Diffusion anisotropy indexes are sensitive to selecting the EPI readout-encoding bandwidth at high-field MRI [published online ahead of print 2008/04/19]. Magn Reson Imaging. 2008. 26(5):676–682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion MRI Connections in the Octopus Brain
- The Role of Double Inversion Recovery Imaging in Acute Ischemic Stroke
- Neuroactivation studies using Functional Brain MRI
- Diffusion-weighted Imaging and Apparent Diffusion Coefficient Maps for the Evaluation of Pyogenic Ventriculitis
- Visual recovery demonstrated by functional MRI and diffusion tensor tractography in bilateral occipital lobe infarction