J Periodontal Implant Sci.
2012 Dec;42(6):224-230.
In vitro assay for osteoinductive activity of different demineralized freeze-dried bone allograft
- Affiliations
-
- 1Department of Periodontology, Dental school, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2Department of Dental Material, Dental school, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 3Department of Periodontology, Dental school, Qazvin University of Medical Sciences, Qazvin, Iran. somayeh_hemat@yahoo.com
Abstract
- PURPOSE
Various bone graft materials have been used for periodontal tissue regeneration. Demineralized freeze-dried bone allograft (DFDBA) is a widely used bone substitute. The current widespread use of DFDBA is based on its potential osteoinductive ability. Due to the lack of verifiable data, the purpose of this study was to assess the osteoinductive activity of different DFDBAs in vitro.
METHODS
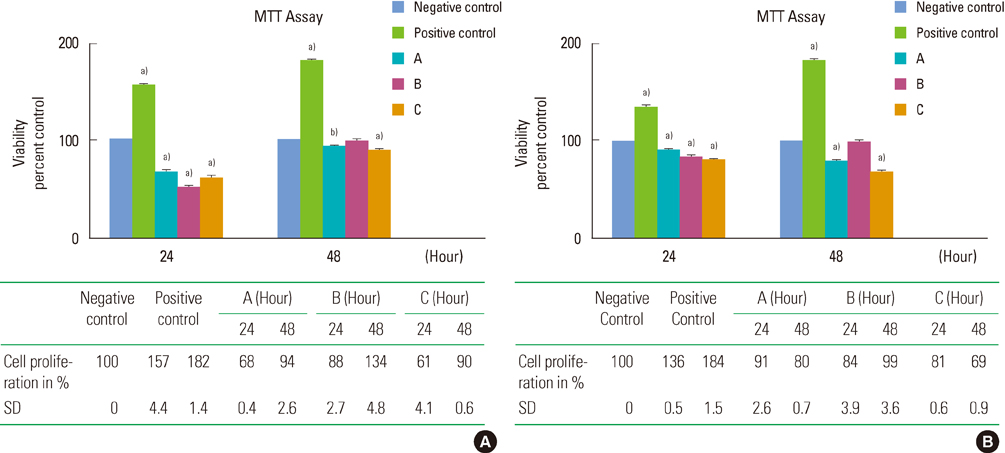
Sarcoma osteogenic (SaOS-2) cells (human osteoblast-like cells) were exposed to 8 mg/mL and 16 mg/mL concentrations of three commercial types of DFDBA: Osseo+, AlloOss, and Cenobone. The effect of these materials on cell proliferation was determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. The osteoinductive ability was evaluated using alizarin red staining, and the results were confirmed by evaluating osteogenic gene expression using reverse transcription polymerase chain reaction (RT-PCR).
RESULTS
In the SaOS-2 cells, an 8 mg/mL concentration of Osseo+ and Cenobone significantly increased cell proliferation in 48 hours after exposure (P<0.001); however, in these two bone materials, the proliferation of cells was significantly decreased after 48 hours of exposure with a 16 mg/mL concentration (P<0.001). The alizarin red staining results demonstrated that the 16 mg/mL concentration of all three tested DFDBA induced complete morphologic differentiation and mineralized nodule production of the SaOS-2 cells. The RT-PCR results revealed osteopontin gene expression at a 16 mg/mL concentration of all three test groups, but not at an 8 mg/mL concentration.
CONCLUSIONS
These commercial types of DFDBA are capable of decreasing proliferation and increasing osteogenic differentiation of the SaOS-2 cell line and have osteoinductive activity in vitro.
MeSH Terms
Figure
Reference
-
1. Urist MR. Bone histogenesis and morphogenesis in implants of demineralized enamel and dentin. J Oral Surg. 1971. 29:88–102.2. Schwartz Z, Mellonig JT, Carnes DL Jr, de la Fontaine J, Cochran DL, Dean DD, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1996. 67:918–926.
Article3. Bormann N, Pruss A, Schmidmaier G, Wildemann B. In vitro testing of the osteoinductive potential of different bony allograft preparations. Arch Orthop Trauma Surg. 2010. 130:143–149.
Article4. Zhang M, Powers RM Jr, Wolfinbarger L Jr. A quantitative assessment of osteoinductivity of human demineralized bone matrix. J Periodontol. 1997. 68:1076–1084.
Article5. Shigeyama Y, D'Errico JA, Stone R, Somerman MJ. Commercially-prepared allograft material has biological activity in vitro. J Periodontol. 1995. 66:478–487.
Article6. Pinholt EM, Haanaes HR, Roervik M, Donath K, Bang G. Alveolar ridge augmentation by osteoinductive materials in goats. Scand J Dent Res. 1992. 100:361–365.
Article7. Becker W, Lynch SE, Lekholm U, Becker BE, Caffesse R, Donath K, et al. A comparison of ePTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. J Periodontol. 1992. 63:929–940.
Article8. Becker W, Schenk R, Higuchi K, Lekholm U, Becker BE. Variations in bone regeneration adjacent to implants augmented with barrier membranes alone or with demineralized freeze-dried bone or autologous grafts: a study in dogs. Int J Oral Maxillofac Implants. 1995. 10:143–154.9. Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989. 60:655–663.
Article10. Schwartz Z, Somers A, Mellonig JT, Carnes DL Jr, Wozney JM, Dean DD, et al. Addition of human recombinant bone morphogenetic protein-2 to inactive commercial human demineralized freeze-dried bone allograft makes an effective composite bone inductive implant material. J Periodontol. 1998. 69:1337–1345.
Article11. Becker W, Urist MR, Tucker LM, Becker BE, Ochsenbein C. Human demineralized freeze-dried bone: inadequate induced bone formation in athymic mice. A preliminary report. J Periodontol. 1995. 66:822–828.
Article12. Kumaran ST, Arun KV, Sudarsan S, Talwar A, Srinivasan N. Osteoblast response to commercially available demineralized bone matrices: an in-vitro study. Indian J Dent Res. 2010. 21:3–9.
Article13. Lian JB, Stein GS. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGF beta 1) and hormones (vitamin D and glucocorticoids). J Oral Implantol. 1993. 19:95–105.14. Rodan SB, Imai Y, Thiede MA, Wesolowski G, Thompson D, Bar-Shavit Z, et al. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987. 47:4961–4966.15. Carnes DL Jr, De La Fontaine J, Cochran DL, Mellonig JT, Keogh B, Harris SE, et al. Evaluation of 2 novel approaches for assessing the ability of demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1999. 70:353–363.
Article16. Rochet N, Loubat A, Laugier JP, Hofman P, Bouler JM, Daculsi G, et al. Modification of gene expression induced in human osteogenic and osteosarcoma cells by culture on a biphasic calcium phosphate bone substitute. Bone. 2003. 32:602–610.
Article17. Trojani C, Weiss P, Michiels JF, Vinatier C, Guicheux J, Daculsi G, et al. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials. 2005. 26:5509–5517.
Article18. Han B, Tang B, Nimni ME. Quantitative and sensitive in vitro assay for osteoinductive activity of demineralized bone matrix. J Orthop Res. 2003. 21:648–654.
Article19. Carinci F, Piattelli A, Degidi M, Palmieri A, Perrotti V, Scapoli L, et al. Effects of demineralized freeze-dried bone allograft on gene expression of osteoblastlike MG63 cells. Int J Periodontics Restorative Dent. 2007. 27:596–601.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of new bone formation of titanium mesh and demineralized freeze-dried bone
- The Fate of Demineralized Human Osteosarcoma in Rat's Muscle Pouch
- BONE REGENERATION IN COMPOSITE GRAFT OF FREEZE-DRIEDDEMINERALIZED BONE AND HYDROXYLAPATITE IN RABBIT CRANIAL DEFECTS
- The Effect of Demineralized Freeze-Dried Bone Allograft in Guided Bone Regeneration on Supra-Alveolar Peri-Implant Defects in Dogs
- The effect of the freeze dried bone allograft and gel/putty type demineralized bone matrix on osseous regeneration in the rat calvarial defects