Allergy Asthma Immunol Res.
2019 Mar;11(2):291-298. 10.4168/aair.2019.11.2.291.
Higher Binding Affinity and In Vitro Potency of Reslizumab for Interleukin-5 Compared With Mepolizumab
- Affiliations
-
- 1R&D, Biologics Lead Antibody Discovery, Teva Pharmaceuticals Australia, Sydney, NSW, Australia. mark.liddament@tevapharm.com
- 2R&D, Biologics, Teva Pharmaceuticals USA, West Chester, PA, USA.
- KMID: 2431860
- DOI: http://doi.org/10.4168/aair.2019.11.2.291
Abstract
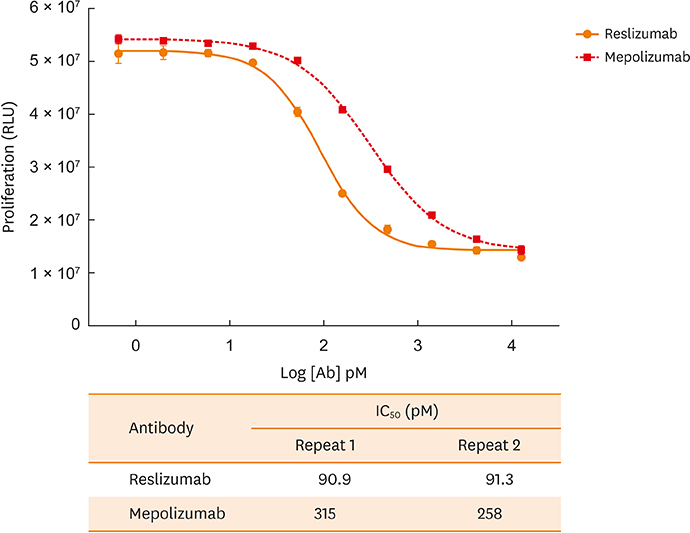
- Reslizumab and mepolizumab are recently approved monoclonal antibodies for the treatment of severe (uncontrolled) eosinophilic asthma. Both are effective in neutralizing the function of interleukin-5 (IL-5). This study is the first to compare the binding affinity and in vitro potency of both antibodies in head-to-head assays. Two assays assessed binding affinity (using the equilibrium dissociation constant [K(D)]) of each drug for human IL-5. In the Biacore surface plasmon resonance assay, the association constant (k(on)) values for human IL-5 for reslizumab and mepolizumab were 3.93 × 10ⶠand 1.83 × 10âµ, respectively. The dissociation constant (k(off)) values were 4.29 × 10â»â´ and 2.14 × 10â»â´, respectively. Calculated K(D) values for human IL-5 for reslizumab and mepolizumab were 109 and 1,170 pM, respectively, representing an approximately 11-fold stronger binding affinity with reslizumab. In the Kinetic Exclusion Assay, the k(on) values for human IL-5 for reslizumab and mepolizumab were 3.17 × 10ⶠand 1.32 × 10âµ, respectively. The k(off) values were 1.36 × 10â»âµ and 1.48 × 10â»âµ, respectively. Measured K(D) values for human IL-5 for reslizumab and mepolizumab were 4.3 and 112 pM, respectively, representing an approximately 26-fold stronger binding affinity for reslizumab. A human-IL-5-dependent cell proliferation assay was developed to assess in vitro potency, based on a human cell line selected for enhanced surface expression of IL-5 receptor-alpha and consistent proliferation response to IL-5. The concentration at which 50% inhibition occurred (ICâ‚…â‚€) was determined for both antibodies. Reslizumab and mepolizumab inhibited IL-5-dependent cell proliferation, with ICâ‚…â‚€ values of approximately 91.1 and 286.5 pM, respectively, representing on average 3.1-fold higher potency with reslizumab. In conclusion, comparative assays show that reslizumab has higher affinity binding for and in vitro potency against human IL-5 compared with mepolizumab. However, these results do not take into consideration the different methods of administration of reslizumab and mepolizumab.
MeSH Terms
Figure
Cited by 1 articles
-
Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity
Wan Yin Winnie Yeung, Hae Sim Park
Yonsei Med J. 2020;61(1):4-14. doi: 10.3349/ymj.2020.61.1.4.
Reference
-
1. Aleman F, Lim HF, Nair P. Eosinophilic endotype of asthma. Immunol Allergy Clin North Am. 2016; 36:559–568.
Article2. Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014; 2:741–750.
Article3. Ulrik CS. Peripheral eosinophil counts as a marker of disease activity in intrinsic and extrinsic asthma. Clin Exp Allergy. 1995; 25:820–827.
Article4. Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, et al. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988; 167:43–56.
Article5. Warringa RA, Schweizer RC, Maikoe T, Kuijper PH, Bruijnzeel PL, Koendermann L. Modulation of eosinophil chemotaxis by interleukin-5. Am J Respir Cell Mol Biol. 1992; 7:631–636.
Article6. Carlson M, Peterson C, Venge P. The influence of IL-3, IL-5, and GM-CSF on normal human eosinophil and neutrophil C3b-induced degranulation. Allergy. 1993; 48:437–442.7. Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstjens HA, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003; 167:1655–1659.8. Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin Pharmacokinet. 2011; 50:215–227.
Article9. Teva Respiratory, LLC (US). CINQAIR®: highlights of prescribing information [Internet]. Frazer (PA): Teva Respiratory, LLC;2016. cited 2017 Oct 26. Available from: http://cinqair.com/pdf/PrescribingInformation.pdf.10. GlaxoSmithKline. NUCALA: highlights of prescribing information [Internet]. Philadelphia (PA): GlaxoSmithKline;2017. cited 2018 Apr 5. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.PDF.11. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015; 3:355–366.
Article12. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009; 360:973–984.
Article13. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659.
Article14. Cabon Y, Molinari N, Marin G, Vachier I, Gamez AS, Chanez P, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017; 47:129–138.
Article15. Drake AW, Tang ML, Papalia GA, Landes G, Haak-Frendscho M, Klakamp SL. Biacore surface matrix effects on the binding kinetics and affinity of an antigen/antibody complex. Anal Biochem. 2012; 429:58–69.
Article16. Jaworowicz D, Fiedler-Kelly J, Rabinovich-Guilatt L, Bond M. The steady-state pharmacokinetic (pk) profile across a range of patient body weight categories supports weight-based dosing for intravenous (iv) reslizumab. Am J Respir Crit Care Med. 2016; 193:A1389.17. Alexander AG, Barkans J, Moqbel R, Barnes NC, Kay AB, Corrigan CJ. Serum interleukin 5 concentrations in atopic and non-atopic patients with glucocorticoid-dependent chronic severe asthma. Thorax. 1994; 49:1231–1233.
Article18. Huang CD, Wang CH, Liu CY, Lin SM, Chou CL, Liu WT, et al. Eosinophils from asthmatics release IL-5 in an autocrine fashion to prevent apoptosis through upregulation of Bcl-2 expression. J Asthma. 2005; 42:395–403.
Article19. Bates JT, Keefer CJ, Utley TJ, Correia BE, Schief WR, Crowe JE Jr. Reversion of somatic mutations of the respiratory syncytial virus-specific human monoclonal antibody Fab19 reveal a direct relationship between association rate and neutralizing potency. J Immunol. 2013; 190:3732–3739.
Article20. European Medicines Agency. ICH topic Q 6 B. Specifications: test procedures and acceptance criteria for biotechnological/biological products [Internet]. London: European Medicines Agency;1999. cited 2018 Apr 11. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002824.pdf.21. Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016; 150:789–798.22. Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016; 150:799–810.23. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014; 371:1198–1207.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Refractory Bullous Pemphigoid Successfully Treated with Reslizumab: A Possible Novel Therapeutic Modality
- Potency and plasma protein binding of drugs in vitro—a potentially misleading pair for predicting in vivo efficacious concentrations in humans
- Two Cases of DRESS Syndrome Successfully Treated with Reslizumab
- Lectin Histochemistry for Effects of N - Nitrosodimethylamine on Glycoconjugates in the Rat Lingual Salivary Glands
- Eosinophils and childhood asthma