J Korean Soc Transplant.
2018 Dec;32(4):75-83. 10.4285/jkstn.2018.32.4.75.
Monitoring of Mycophenolic Acid Trough Concentration in Kidney Transplant under Cyclosporine Is Beneficial in Reducing Acute Rejection within 1 Year
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kmhyj.kim@samsung.com
- KMID: 2430587
- DOI: http://doi.org/10.4285/jkstn.2018.32.4.75
Abstract
- BACKGROUND
This study was designed to analyze the clinical usefulness of mycophenolic acid trough concentration monitoring in kidney transplantation patients who were maintained with cyclosporine.
METHODS
The data of patients who underwent mycophenolic acid trough concentration monitoring after their first kidney transplant between November 2006 and August 2013 and were prescribed with cyclosporine, mycophenolate, and methylprednisolone were reviewed retrospectively. Cox analysis was used to analyze the risk factors for acute rejection within 1 year post-transplantation.
RESULTS
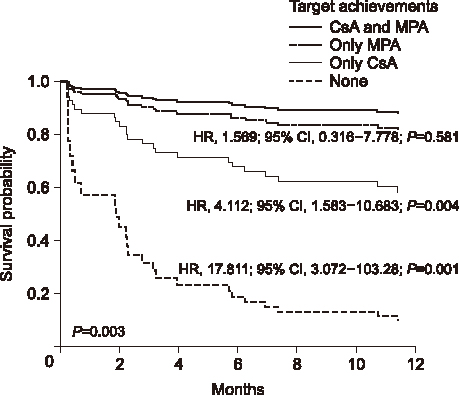
Among 90 patients, 41 (45.6%) achieved both the target levels of cyclosporine and mycophenolic acid, while three patients (3.3%) failed to achieve the target level of either cyclosporine or mycophenolic acid. Nine patients (10.0%) only achieved the mycophenolic acid target level and 37 patients (41.1%) only achieved the cyclosporine target level. While patients who achieved only the mycophenolic acid target concentration had no statistically increased risk compared to patients who achieved both target levels (hazard ratio [HR], 1.569; 95% confidence interval [CI], 0.316 to 7.778; P=0.581), patients who only achieved the cyclosporine target concentration showed an increased risk of rejection compared to the both achievement group (HR, 4.112; 95% CI, 1.583 to 10.683; P=0.004). Patients who had no achievement in the target levels showed significantly increased rejection risk compared to the patients who achieved both target levels (HR, 17.811; 95% CI, 3.072 to 103.28; P=0.001).
CONCLUSIONS
Mycophenolic acid trough concentration monitoring combined with cyclosporine trough concentration monitoring is useful for avoiding acute cellular rejection if the first 1 year post-transplantation.
MeSH Terms
Figure
Reference
-
1. Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009; 87:785–794.
Article2. Bunnapradist S, Sampaio MS, Wilkinson AH, Pham PT, Huang E, Kuo HT, et al. Changes in the small bowel of symptomatic kidney transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium. Am J Nephrol. 2014; 40:184–190.
Article3. Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998; 34:429–455.
Article4. Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000; 47:215–245.
Article5. Wagner M, Earley AK, Webster AC, Schmid CH, Balk EM, Uhlig K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2015; 12:CD007746.
Article6. Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010; 5:341–358.
Article7. van Gelder T, Hesselink DA. Mycophenolate revisited. Transpl Int. 2015; 28:508–515.
Article8. Hale MD, Nicholls AJ, Bullingham RE, Hene R, Hoitsma A, Squifflet JP, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998; 64:672–683.
Article9. van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999; 68:261–266.
Article10. Tett SE, Saint-Marcoux F, Staatz CE, Brunet M, Vinks AA, Miura M, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev (Orlando). 2011; 25:47–57.
Article11. van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006; 28:145–154.
Article12. Weber LT, Hoecker B, Armstrong VW, Oellerich M, Tonshoff B. Validation of an abbreviated pharmacokinetic profile for the estimation of mycophenolic acid exposure in pediatric renal transplant recipients. Ther Drug Monit. 2006; 28:623–631.
Article13. Borrows R, Chusney G, Loucaidou M, James A, Lee J, Tromp JV, et al. Mycophenolic acid 12-h trough level monitoring in renal transplantation: association with acute rejection and toxicity. Am J Transplant. 2006; 6:121–128.
Article14. Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, et al. Long-term changes in mycophenolic acid exposure in combination with tacrolimus and corticosteroids are dose dependent and not reflected by trough plasma concentration: a prospective study in 100 de novo renal allograft recipients. J Clin Pharmacol. 2003; 43:866–880.
Article15. Le Meur Y, Buchler M, Thierry A, Caillard S, Villemain F, Lavaud S, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007; 7:2496–2503.
Article16. Weber LT, Shipkova M, Armstrong VW, Wagner N, Schutz E, Mehls O, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic Acid in pediatric renal transplant recipients: a report of the german study group on mycophenolate mofetil therapy. J Am Soc Nephrol. 2002; 13:759–768.
Article17. Gardiner KM, Tett SE, Staatz CE. Multinational evaluation of mycophenolic acid, tacrolimus, cyclosporin, sirolimus, and everolimus utilization. Ann Transplant. 2016; 21:1–11.
Article18. Axelrod DA, Naik AS, Schnitzler MA, Segev DL, Dharnidharka VR, Brennan DC, et al. National variation in use of immunosuppression for kidney transplantation: a call for evidence-based regimen selection. Am J Transplant. 2016; 16:2453–2462.
Article19. Miura M, Niioka T, Kato S, Kagaya H, Saito M, Habuchi T, et al. Monitoring of mycophenolic acid predose concentrations in the maintenance phase more than one year after renal transplantation. Ther Drug Monit. 2011; 33:295–302.
Article20. Budde K, Bauer S, Hambach P, Hahn U, Roblitz H, Mai I, et al. Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients. Am J Transplant. 2007; 7:888–898.
Article21. Cattaneo D, Cortinovis M, Baldelli S, Bitto A, Gotti E, Remuzzi G, et al. Pharmacokinetics of mycophenolate sodium and comparison with the mofetil formulation in stable kidney transplant recipients. Clin J Am Soc Nephrol. 2007; 2:1147–1155.
Article22. de Winter BC, van Gelder T, Glander P, Cattaneo D, Tedesco-Silva H, Neumann I, et al. Population pharmacokinetics of mycophenolic acid : a comparison between enteric-coated mycophenolate sodium and mycophenolate mofetil in renal transplant recipients. Clin Pharmacokinet. 2008; 47:827–838.23. Rhu J, Lee KW, Park H, Park JB, Kim SJ, Choi GS. Clinical implication of mycophenolic acid trough concentration monitoring in kidney transplant patients on a tacrolimus triple maintenance regimen: a single-center experience. Ann Transplant. 2017; 22:707–718.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Successful Treatment of Cutaneous Aspergillosis with Voriconazole at the Low Cyclosporine Trough Level in a Renal Transplant
- The Safety and Effectiveness of Microemulsion Cyclosporine in Renal Allograft Recipients: 1 Year Follow-Up Study
- Phased Reduction of Cyclosporine Combined with Mycophenolate Mofetil in Renal Transplant Recipients: Three-year Results of a Prospective Study
- Mycophenolic Acid Trough Concentration and Dose Are Associated with Hematologic Abnormalities but Not Rejection in Kidney Transplant Recipients
- The Efficacy and Outcome of Reduced Dose of Tacrolimus in Renal Transplantation