J Korean Med Sci.
2020 Jun;35(24):e185. /10.3346/jkms.2020.35.e185.
Mycophenolic Acid Trough Concentration and Dose Are Associated with Hematologic Abnormalities but Not Rejection in Kidney Transplant Recipients
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Republic of Korea
- 2Department of Internal Medicine, Pohang St. Mary's Hospital, Pohang, Republic of Korea
- 3Department of Statistics, College of Natural Sciences, Kyungpook National University, Daegu, Republic of Korea
- 4Department of Surgery, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Republic of Korea
- 5Department of Clinical Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Republic of Korea
- KMID: 2503013
- DOI: http://doi.org//10.3346/jkms.2020.35.e185
Abstract
- Background
Little is known regarding the safe fixed dose of mycophenolic acid (MPA) for preventing biopsy-proven acute rejection (BPAR) in kidney transplant recipients (KTRs). We investigated the correlation of MPA trough concentration (MPA C0) and dose with renal transplant outcomes and adverse events.
Methods
This study included 79 consecutive KTRs who received MPA with tacrolimus (TAC) and corticosteroids. The MPA C0 of all the enrolled KTRs was measured, which was determined monthly by using particle-enhanced turbidimetric inhibition immunoassay for 12 months, and clinical data were collected at each time point. The clinical endpoints included BPAR, any cytopenia, and BK or cytomegalovirus infections.
Results
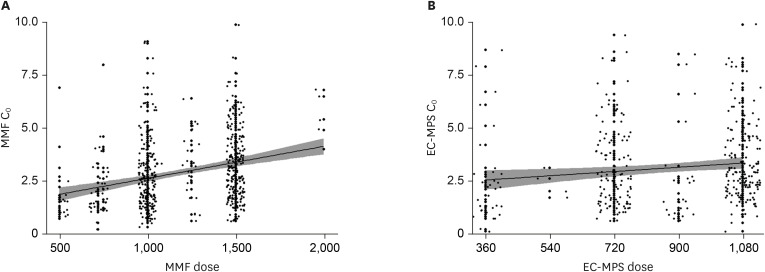
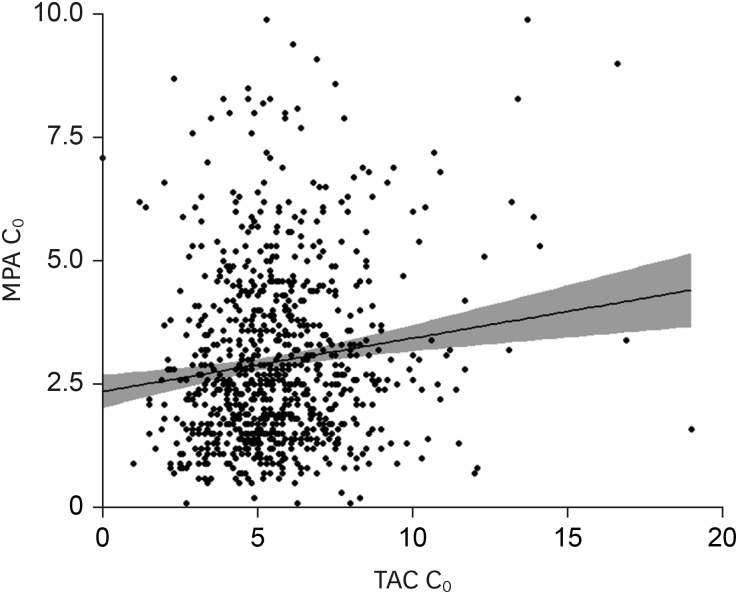
No differences in MPA C0 and dose were observed between KTRs with or without BPAR or viral infections under statistically comparable TAC concentrations. MPA C0 was significantly higher in patients with leukopenia (P = 0.021) and anemia (P = 0.002) compared with those without cytopenia. The MPA dose was significantly higher in patients with thrombocytopenia (P = 0.002) compared with those without thrombocytopenia. MPA C0 ≥ 3.5 μg/mL was an independent risk factor for leukopenia (adjusted odds ratio [AOR], 3.80; 95% confidence interval [CI], 1.24–11.64; P = 0.019) and anemia (AOR, 5.90; 95% CI, 1.27–27.51; P = 0.024). An MPA dose greater than the mean value of 1,188.8 mg/day was an independent risk factor for thrombocytopenia (AOR, 3.83; 95% CI, 1.15–12.78; P = 0.029). However, an MPA dose less than the mean value of 1,137.3 mg/day did not increase the risk of BPAR.
Conclusion
Either a higher MPA C0 or dose is associated with an increased risk of cytopenia, but neither a lower MPA C0 nor dose is associated with BPAR within the first year of transplantation. Hence, a reduced MPA dose with TAC and corticosteroids might be safe in terms of reducing hematologic abnormalities without causing rejection.
Figure
Reference
-
1. Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995; 17(6):681–684. PMID: 8588241.
Article2. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007; 46(1):13–58. PMID: 17201457.
Article3. Shaw LM, Korecka M, Venkataramanan R, Goldberg L, Bloom R, Brayman KL. Mycophenolic acid pharmacodynamics and pharmacokinetics provide a basis for rational monitoring strategies. Am J Transplant. 2003; 3(5):534–542. PMID: 12752309.
Article4. van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999; 68(2):261–266. PMID: 10440399.5. Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain F, Lavaud S, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007; 7(11):2496–2503. PMID: 17908276.
Article6. Mourad M, Malaise J, Chaib Eddour D, De Meyer M, König J, Schepers R, et al. Pharmacokinetic basis for the efficient and safe use of low-dose mycophenolate mofetil in combination with tacrolimus in kidney transplantation. Clin Chem. 2001; 47(7):1241–1248. PMID: 11427455.
Article7. Armstrong VW, Shipkova M, Schütz E, Weber L, Tönshoff B, Oellerich M, et al. Monitoring of mycophenolic acid in pediatric renal transplant recipients. Transplant Proc. 2001; 33(1-2):1040–1043. PMID: 11267182.
Article8. Weber LT, Shipkova M, Armstrong VW, Wagner N, Schütz E, Mehls O, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: a report of the German study group on mycophenolate mofetil therapy. J Am Soc Nephrol. 2002; 13(3):759–768. PMID: 11856782.
Article9. Borrows R, Chusney G, Loucaidou M, James A, Lee J, Tromp JV, et al. Mycophenolic acid 12-h trough level monitoring in renal transplantation: association with acute rejection and toxicity. Am J Transplant. 2006; 6(1):121–128. PMID: 16433766.
Article10. Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, et al. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial. Am J Transplant. 2009; 9(7):1607–1619. PMID: 19459794.
Article11. van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation. 2008; 86(8):1043–1051. PMID: 18946341.
Article12. Ham JY, Jung HY, Choi JY, Park SH, Kim YL, Kim HK, et al. Usefulness of mycophenolic acid monitoring with PETINIA for prediction of adverse events in kidney transplant recipients. Scand J Clin Lab Invest. 2016; 76(4):296–303. PMID: 26981890.
Article13. Jung HY, Cho SY, Choi JY, Cho JH, Park SH, Kim YL, et al. Comparison of transplant outcomes for low-level and standard-level tacrolimus at different time points after kidney transplantation. J Korean Med Sci. 2019; 34(12):e103. PMID: 30940998.
Article14. Rodrigo E, Segundo DS, Fernández-Fresnedo G, López-Hoyos M, Benito A, Ruiz JC, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation. 2016; 100(11):2479–2485. PMID: 26703349.
Article15. Vanichanan J, Udomkarnjananun S, Avihingsanon Y, Jutivorakool K. Common viral infections in kidney transplant recipients. Kidney Res Clin Pract. 2018; 37(4):323–337. PMID: 30619688.
Article16. Doria C, Greenstein S, Narayanan M, Ueda K, Wiland A, McCague K, et al. Association of mycophenolic acid dose with efficacy and safety events in kidney transplant patients receiving tacrolimus: an analysis of the Mycophenolic acid Observational REnal transplant registry. Clin Transplant. 2012; 26(6):E602–11. PMID: 23121178.
Article17. Lee SH, Kim CD, Huh KH, Cho BH, Ju MK, Lee DR, et al. Low-dose mycophenolate mofetil in tablet form or capsule form combined with tacrolimus in the early period after kidney transplantation: a prospective randomized trial. Clin Nephrol. 2016; 86 (2016)(12):319–327. PMID: 27781419.
Article18. Rhu J, Lee KW, Park H, Park JB, Kim SJ, Choi GS. Clinical implication of mycophenolic acid trough concentration monitoring in kidney transplant patients on a tacrolimus triple maintenance regimen: a single-center experience. Ann Transplant. 2017; 22:707–718. PMID: 29180612.
Article19. Cattaneo D, Cortinovis M, Baldelli S, Bitto A, Gotti E, Remuzzi G, et al. Pharmacokinetics of mycophenolate sodium and comparison with the mofetil formulation in stable kidney transplant recipients. Clin J Am Soc Nephrol. 2007; 2(6):1147–1155. PMID: 17928466.
Article20. de Winter BC, van Gelder T, Glander P, Cattaneo D, Tedesco-Silva H, Neumann I, et al. Population pharmacokinetics of mycophenolic acid : a comparison between enteric-coated mycophenolate sodium and mycophenolate mofetil in renal transplant recipients. Clin Pharmacokinet. 2008; 47(12):827–838. PMID: 19026038.21. Weber LT, Shipkova M, Lamersdorf T, Niedmann PD, Wiesel M, Mandelbaum A, et al. Pharmacokinetics of mycophenolic acid (MPA) and determinants of MPA free fraction in pediatric and adult renal transplant recipients. German study group on mycophenolate mofetil therapy in pediatric renal transplant recipients. J Am Soc Nephrol. 1998; 9(8):1511–1520. PMID: 9697675.
Article22. Atcheson BA, Taylor PJ, Kirkpatrick CM, Duffull SB, Mudge DW, Pillans PI, et al. Free mycophenolic acid should be monitored in renal transplant recipients with hypoalbuminemia. Ther Drug Monit. 2004; 26(3):284–286. PMID: 15167629.
Article23. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009; 48(3):143–157. PMID: 19385708.
Article24. Jones H, Talwar M, Nogueira JM, Ugarte R, Cangro C, Rasheed H, et al. Anemia after kidney transplantation; its prevalence, risk factors, and independent association with graft and patient survival: a time-varying analysis. Transplantation. 2012; 93(9):923–928. PMID: 22377790.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring of Mycophenolic Acid Trough Concentration in Kidney Transplant under Cyclosporine Is Beneficial in Reducing Acute Rejection within 1 Year
- A Pilot Study of Calcineurin Inhibitors (CNIs) and Steroid Avoidance Immunosuppressive Protocol among Living Donor Kidney Transplant Recipients
- A Concept Analysis of Compliance in Kidney Transplant Recipient Including Compliance with Immunosuppressive Medication
- Pharmacokinetic Study of Mycophenolic Acid in Korean Kidney Transplant Patients
- The Efficacy and Outcome of Reduced Dose of Tacrolimus in Renal Transplantation