Korean J Radiol.
2019 Jan;20(1):148-157. 10.3348/kjr.2017.0771.
Management of Adverse Reactions to Iodinated Contrast Media for Computed Tomography in Korean Referral Hospitals: A Survey Investigation
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul, Korea. yshoka@gmail.com
- 2Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea.
- 3Drug Safety Monitoring Center, Seoul National University Hospital, Seoul, Korea.
- 4Seoul National University Hospital Regional Pharmacovigilance Center, Seoul, Korea.
- 5Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2429928
- DOI: http://doi.org/10.3348/kjr.2017.0771
Abstract
OBJECTIVE
To evaluate the current status of managing adverse reactions to iodinated contrast media (ICM) for computed tomography in referral hospitals in South Korea compared with hospitals in other countries.
MATERIALS AND METHODS
This survey investigation involved 59 Korean and 15 overseas hospitals using guideline-based questionnaires consisting of 24 items in 7 main categories related to managing adverse reactions to ICM.
RESULTS
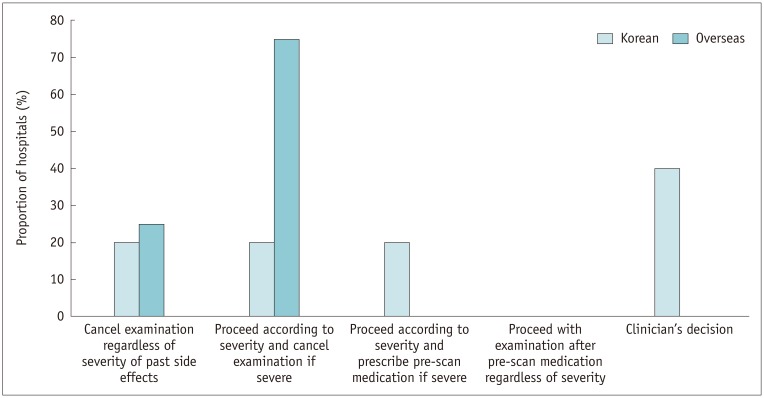
Informed written consent with risk factor evaluation was appropriately performed in most of the Korean hospitals. There was considerable variability in assessing renal function across the hospitals; serum creatinine level was used as a reference in 76.4% of Korean hospitals. The Korean hospitals preferred a more stringent approach to determining normal renal function (p = 0.01), withholding metformin (p = 0.01), and fasting before ICM exposure (p < 0.001) compared with overseas hospitals. All the Korean hospitals had an emergency protocol and in-hospital system for adverse reactions to ICM. The Korean (87.7%) and overseas hospitals (100%) were similarly equipped with epinephrine (p = 0.332), but only 38.6% of Korean hospitals were equipped with a bronchodilator (p = 0.004). For patients with a previous hypersensitivity reaction to ICM, 62.3% of Korean hospitals pre-medicated with anti-histamine and corticosteroid according to the severity of the previous reaction, and changed the culprit ICM in 52.8%, while skin test was performed in 17%.
CONCLUSION
In general, Korean referral hospitals were well-prepared regarding informed consent, protocol, and an in-hospital system for managing adverse reactions to ICM. Nevertheless, there was considerable variability in details and management, thus requiring standardization by reflecting current guidelines.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Metformin Treatment for Patients with Diabetes and Chronic Kidney Disease: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
Kyu Yeon Hur, Mee Kyoung Kim, Seung Hyun Ko, Miyeun Han, Dong Won Lee, Hyuk-Sang Kwon, ,
Diabetes Metab J. 2020;44(1):3-10. doi: 10.4093/dmj.2020.0004.Preparative fasting before contrast-enhanced computed tomography
Hwan Seok Yong
J Korean Med Assoc. 2020;63(3):151-154. doi: 10.5124/jkma.2020.63.3.151.Change of culprit agent prevents recurrent hypersensitivity reactions to iodinated contrast media
Min Jae Cha, Whal Lee
J Korean Med Assoc. 2020;63(3):145-150. doi: 10.5124/jkma.2020.63.3.145.
Reference
-
1. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013; 22:149–154. PMID: 24436602.
Article2. Bae K, Lee SM, Ha JY, Jeon KN, Moon JI, Choi BH, et al. Adverse drug reactions to CT contrast media in South Korea: incidence and risk factors. J Korean Soc Radiol. 2016; 75:41–48.
Article3. Bata P, Tarnoki AD, Tarnoki DL, Horvath E, Berczi V, Szalay F. Acute severe thrombocytopenia following non-ionic low-osmolarity intravenous contrast medium injection. Korean J Radiol. 2012; 13:505–509. PMID: 22778575.
Article4. UCSF Department of Radiology & Biomedical imaging Web site. CT and X-ray contrast guidelines. Updated July 20, 2017. Accessed September 17, 2017. https://radiology.ucsf.edu/patient-care/patient-safety/contrast/iodinated.5. ACR Committee on Drugs and ContrastMedia. ACR manual on contrast media version 10.3. 2017. Accessed September 17, 2017. Available at: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.6. Korean Society of Radiology. Korean Academy of Asthma. KIoDSRM. Korean clinical practice guideline on adverse reactions of injectable iodinated contrast agent and gadolinium contrast agent for MRI, 2nd edition. Accessed September 17, 2017. Available at: http://www.allergy.or.kr/file/allergic2016.pdf. [Published 2016].7. Korean Institute of Drug Safety & Risk Management imaging Web site. Drug safety information report trend. Accessed September 17, 2017. http://open.drugsafe.or.kr/trend/trend/Read.jsp?ntt_id=2003. Published 2016.8. ESUR guidelines on contrast media. Version 8.1. European Society of Urogenital Radiology Web site. Accessed September 17, 2017. http://www.esur.org/guidelines/. Published 2013.9. Lee J, Cho JY, Lee HJ, Jeong YY, Kim CK, Park BK, et al. Korean Society of Urogenital Radiology (KSUR). Korean Society of Radiology. Contrast-induced nephropathy in patients undergoing intravenous contrast-enhanced computed tomography in Korea: a multi-institutional study in 101487 patients. Korean J Radiol. 2014; 15:456–446. PMID: 25053905.
Article10. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013; 268:719–728. PMID: 23579046.
Article11. Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010; (4):CD002967.
Article12. Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care. 1999; 22:925–927. PMID: 10372243.
Article13. Oowaki K, Saigusa H, Ojiri H, Ariizumi M, Yamagisi J, Fukuda K, et al. [Relationship between oral food intake and nausea caused by intravenous injection of iodinated contrast material]. Nihon Igaku Hoshasen Gakkai Zasshi. 1994; 54:476–479. PMID: 8028954.14. Wagner HJ, Evers JP, Hoppe M, Klose KJ. [Must the patient fast before intravascular injection of a non-ionic contrast medium? Results of a controlled study]. Rofo. 1997; 166:370–375. PMID: 9198507.15. Webb JA, Stacul F, Thomsen HS, Morcos SK. Members Of The Contrast Media Safety Committee Of The European Society Of Urogenital Radiology. Late adverse reactions to intravascular iodinated contrast media. Eur Radiol. 2003; 13:181–184. PMID: 12541128.16. Loh S, Bagheri S, Katzberg RW, Fung MA, Li CS. Delayed adverse reaction to contrast-enhanced CT: a prospective single-center study comparison to control group without enhancement. Radiology. 2010; 255:764–771. PMID: 20406882.
Article17. Abe S, Fukuda H, Tobe K, Ibukuro K. Protective effect against repeat adverse reactions to iodinated contrast medium: premedication vs. changing the contrast medium. Eur Radiol. 2016; 26:2148–2154. PMID: 26427700.
Article18. Park HJ, Park JW, Yang MS, Kim MY, Kim SH, Jang GC, et al. Re-exposure to low osmolar iodinated contrast media in patients with prior moderate-to-severe hypersensitivity reactions: a multicentre retrospective cohort study. Eur Radiol. 2017; 27:2886–2893. PMID: 27975150.
Article19. Lee SY, Yang MS, Choi YH, Park CM, Park HW, Cho SH, et al. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann Allergy Asthma Immunol. 2017; 118:339–344.e1. PMID: 28087383.
Article20. Yoon SH, Lee SY, Kang HR, Kim JY, Hahn S, Park CM, et al. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: a meta-analysis. Allergy. 2015; 70:625–637. PMID: 25649510.
Article21. Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate allergic reactions to gadolinium-based contrast agents: a systematic review and meta-analysis. Radiology. 2018; 286:471–482. PMID: 28846495.
Article22. Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG Jr, Froelich JW, et al. ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007; 188:1447–1474. PMID: 17515363.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transient Orbitofacial Angioedema due to Intravenous Iodinated Contrast Media During Computed Tomography: CT Findings
- Management of adverse reaction to iodinated radiocontrast media
- Risk Factors for Adverse Reactions to Iodinated Contrast Media in Computed Tomography
- Late adverse reactions to iopromide (Ultravist(R)) diagnosed by the patch test: a case report
- Prescreening skin test effectiveness in predicting hypersensitivity to iodinated contrast media