Ann Dermatol.
2016 Feb;28(1):65-73. 10.5021/ad.2016.28.1.65.
Topical Products for Human Hair Regeneration: A Comparative Study on an Animal Model
- Affiliations
-
- 1Department of Pathophysiology, Physiological Sciences Division, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
- 2Department of Biomedical Sciences, Ross University School of Veterinary Medicine, Basseterre, St. Kitts, West Indies. pompeibolfa@gmail.com
- 3Department of Pathology, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania.
- 4Department of Histology, Morphological Sciences Division, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
- 5Department of Histology, Physiological Sciences Division, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
- KMID: 2429483
- DOI: http://doi.org/10.5021/ad.2016.28.1.65
Abstract
- BACKGROUND
Hair loss and hair growth is the subject of tremendous amount of research.
OBJECTIVE
This study investigated the efficacy of three chemical treatments used in humans for hair loss, using a rat model of hair regrowth. The products tested were 2% minoxidil, Hairgrow (Dar-Al-Dawa Pharma), Aminexil, Dercos (Vichy Laboratoires), and Kerium, Anti-chute (La Roche-Posay).
METHODS
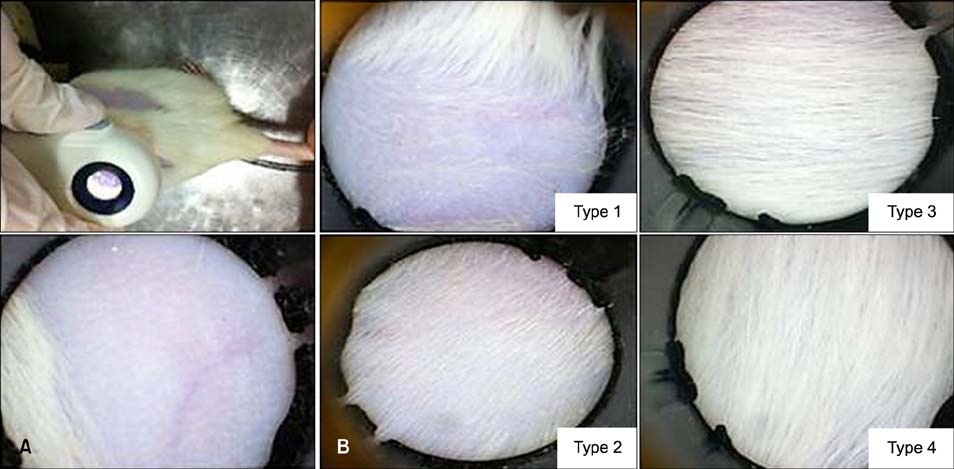
Thirty-two adult female Wistar-Bratislava rats were assigned to 4 groups. Two rectangular areas (2x4 cm) were shaved on either sides of the mid dorsal line (left side - control; right side - test area). Group I was treated topically with 2% minoxidil, group II with Aminexil, and group III with Kerium. Each rat received 0.3 ml of substance applied topically to the shaved dorsal skin every day for 28 days. Rats in group IV served as sham controls receiving no treatment. Hair regrowth was evaluated by trichoscopy (with a dermatoscope), grown hair weight (from a surface area of 1 cm2), and histopathological examination for skin thickness, follicle count, and percentage of anagen induction (morphometric assessment).
RESULTS
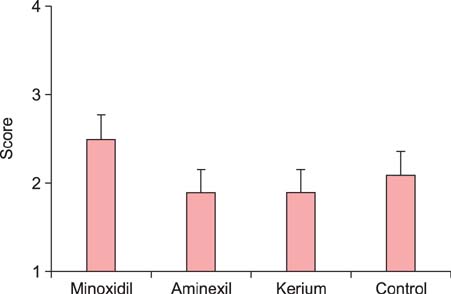
Treatment with 2% minoxidil significantly induced hair regrowth as assessed by trichoscopy, hair weight examination, and morphometric evaluation. Hair weight examination and morphometric assessment demonstrated the lowest hair growth effect with Aminexil among the tested products. Treatment with Kerium was found to significantly induce hair regrowth (p<0.05 as compared to the control group).
CONCLUSION
Our study demonstrates that hair regrowth efficacy of products recommended for human use is not similar when tested on an animal model.
Keyword
Figure
Reference
-
1. Cash TF. The psychology of hair loss and its implications for patient care. Clin Dermatol. 2001; 19:161–166.
Article2. Stough D, Stenn K, Haber R, Parsley WM, Vogel JE, Whiting DA, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin Proc. 2005; 80:1316–1322.
Article3. Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004; 123:455–457.
Article4. Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993; 29:568–575.
Article5. Poulos GA, Mirmirani P. Investigational medications in the treatment of alopecia. Expert Opin Investig Drugs. 2005; 14:177–184.
Article6. Chase HB. Growth of the hair. Physiol Rev. 1954; 34:113–126.
Article7. Johnson E, Ebling FJ. The effect of plucking hairs during different phases of the follicular cycle. J Embryol Exp Morphol. 1964; 12:465–474.
Article8. Hynd PI, Schlink AC, Phillips PM, Scobie DR. Mitotic activity in cells of the wool follicle bulb. Aust J Biol Sci. 1986; 39:329–339.
Article9. Uno H. Quantitative models for the study of hair growth in vivo. Ann N Y Acad Sci. 1991; 642:107–124.10. Hattori M, Ogawa H. Biochemical analysis of hair growth from the aspects of aging and enzyme activities. J Dermatol. 1983; 10:45–54.
Article11. Sundberg JP, King LE Jr. Mouse models for the study of human hair loss. Dermatol Clin. 1996; 14:619–632.
Article12. Ahmad W, Faiyaz ul, Brancolini V, Tsou HC, ul Haque S, Lam H, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998; 279:720–724.
Article13. Cotsarelis G. Male pattern balding may be due to stem cell inactivation, according to penn study [Home page on the Internet]. Philadelphia: 2011. updated 2011 Jan 4. cited 2012 Sep 12. Available from: http://www.uphs.upenn.edu/news/News_Releases/2011/01/male-pattern-balding-stemcell-inactivation/.14. Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988; 27:8706–8711.
Article15. Moretti G, Rebora A, Giacometti C, Boido V, Rampini E, Cipriani C. The quantitative behavior of cutaneous histamine and mast cells in the hair cycles of rats. J Invest Dermatol. 1966; 46:231–239.
Article16. Gordon KA, Tosti A. Alopecia: evaluation and treatment. Clin Cosmet Investig Dermatol. 2011; 4:101–106.
Article17. Tsuboi R, Tanaka T, Nishikawa T, Ueki R, Yamada H, Katsuoka K, et al. A randomized, placebo-controlled trial of 1% topical minoxidil solution in the treatment of androgenetic alopecia in Japanese women. Eur J Dermatol. 2007; 17:37–44.18. Price VH. Treatment of hair loss. N Engl J Med. 1999; 341:964–973.
Article19. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999; 341:491–497.
Article20. Yoon JI, Al-Reza SM, Kang SC. Hair growth promoting effect of Zizyphus jujuba essential oil. Food Chem Toxicol. 2010; 48:1350–1354.
Article21. Bandaranayake I, Mirmirani P. Hair loss remedies--separating fact from fiction. Cutis. 2004; 73:107–114.22. Paus R. Therapeutic strategies for treating hair loss. Drug Discov Today Ther Strateg. 2006; 3:101–110.
Article23. Angelelli L, Cavina G, Moretti G, Siniscalchi P. Quantitative analysis of phospholipids of biochemical and pharmaceutical interest by means of thin layer chromatography: evaluation of the precision of the method. Farmaco Prat. 1966; 21:493–507.24. Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992; 115:587–593.
Article25. Courtois M, Loussouarn G, Hourseau C, Grollier JF. Ageing and hair cycles. Br J Dermatol. 1995; 132:86–93.
Article26. Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008; 59:547–566. quiz 567-568
Article27. Dry E. The coat of the mouse (Mus musculum). J Genet. 1926; 1:287–340.28. Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. J Drugs Dermatol. 2010; 9:1412–1419.29. Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I, Pandya AG, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004; 50:541–553.
Article30. Olsen EA, DeLong ER, Weiner MS. Long-term follow-up of men with male pattern baldness treated with topical minoxidil. J Am Acad Dermatol. 1987; 16:688–695.
Article31. Uno H, Kurata S. Chemical agents and peptides affect hair growth. J Invest Dermatol. 1993; 101:1 Suppl. 143S–147S.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of animal models in biomedical research: a review
- A Survey of the Awareness, Knowledge and Behavior of Hair Dye Use in a Korean Population with Gray Hair
- Hair Cell Regeneration: From Animals to Humans
- Four Cases of Hair Shaft Breakage Caused by Hair Care Cosmetics
- Vestibular Hair Cell Regeneration in Guinea Pig after Gentamicin Damage