Ann Lab Med.
2018 Nov;38(6):578-584. 10.3343/alm.2018.38.6.578.
Development of a Rapid Automated Fluorescent Lateral Flow Immunoassay to Detect Hepatitis B Surface Antigen (HBsAg), Antibody to HBsAg, and Antibody to Hepatitis C
- Affiliations
-
- 1Department of Molecular & Cell Biology, Graduate School, The Catholic University of Korea, Seoul, Korea.
- 2Department of Laboratory Medicine, College of Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. ejoh@catholic.ac.kr, yonggoo@catholic.ac.kr
- 3Central Lab, R&D Center, Boditech Med, Chungcheon, Korea.
- 4Department of Laboratory Medicine, International St. Mary's Hospital, College of Medicine, Catholic Kwandong University, Incheon, Korea.
- 5Department of Biomedical Science, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea.
- KMID: 2429124
- DOI: http://doi.org/10.3343/alm.2018.38.6.578
Abstract
- BACKGROUND
Accurate, rapid, and cost-effective screening tests for hepatitis B virus (HBV) and hepatitis C virus (HCV) infection may be useful in laboratories that cannot afford automated chemiluminescent immunoassays (CLIAs). We evaluated the diagnostic performance of a novel rapid automated fluorescent lateral flow immunoassay (LFIA).
METHODS
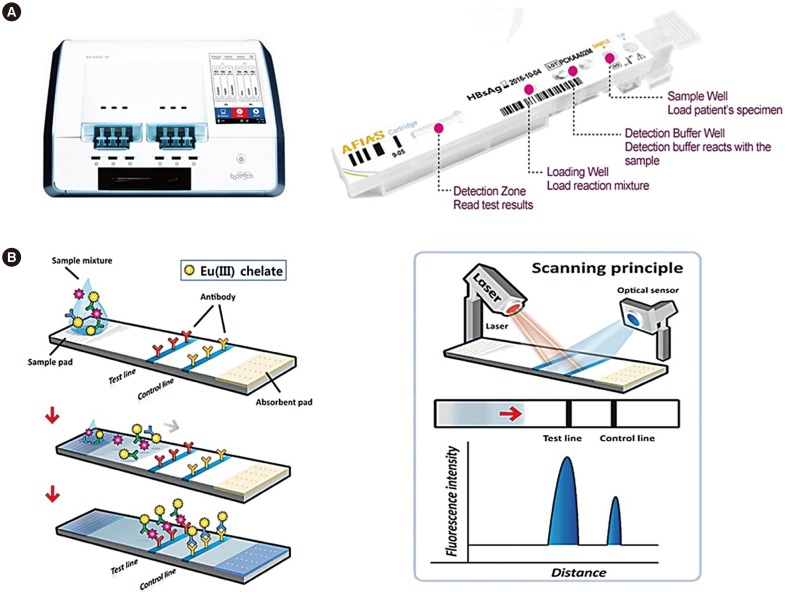
A fluorescent LFIA using a small bench-top fluorescence reader, Automated Fluorescent Immunoassay System (AFIAS; Boditech Med Inc., Chuncheon, Korea), was developed for qualitative detection of hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti-HBs), and antibody to HCV (anti-HCV) within 20 minutes. We compared the diagnostic performance of AFIAS with that of automated CLIAs"”Elecsys (Roche Diagnostics GmbH, Penzberg, Germany) and ARCHITECT (Abbott Laboratories, Abbott Park, IL, USA)"”using 20 seroconversion panels and 3,500 clinical serum samples.
RESULTS
Evaluation with the seroconversion panels demonstrated that AFIAS had adequate sensitivity for HBsAg and anti-HCV detection. From the clinical samples, AFIAS sensitivity and specificity were 99.8% and 99.3% for the HBsAg test, 100.0% and 100.0% for the anti-HBs test, and 98.8% and 99.1% for the anti-HCV test, respectively. Its agreement rates with the Elecsys HBsAg, anti-HBs, and anti-HCV detection assays were 99.4%, 100.0%, and 99.0%, respectively. AFIAS detected all samples with HBsAg genotypes A-F and H and anti-HCV genotypes 1, 1a, 1b, 2a, 2b, 4, and 6. Cross-reactivity with other infections was not observed.
CONCLUSIONS
The AFIAS HBsAg, anti-HBs, and anti-HCV tests demonstrated diagnostic performance equivalent to current automated CLIAs. AFIAS could be used for a large-scale HBV or HCV screening in low-resource laboratories or low-to middle-income areas.
MeSH Terms
Figure
Cited by 1 articles
-
인플루엔자 진단을 위한 두 가지 신속검사의 평가
Jin Ju Kim, Yonggeun Cho, Sang-Guk Lee
Lab Med Online. 2020;10(2):160-164. doi: 10.3343/lmo.2020.10.2.160.
Reference
-
1. Shivkumar S, Peeling R, Jafari Y, Joseph L, Pant Pai N. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012; 157:558–566. PMID: 23070489.2. O'Connell RJ, Gates RG, Bautista CT, Imbach M, Eggleston JC, Beardsley SG, et al. Laboratory evaluation of rapid test kits to detect hepatitis C antibody for use in predonation screening in emergency settings. Transfusion. 2013; 53:505–517. PMID: 22823283.3. Njai HF, Shimakawa Y, Sanneh B, Ferguson L, Ndow G, Mendy M, et al. Validation of rapid point-of-care (POC) tests for detection of hepatitis B surface antigen in field and laboratory settings in the Gambia, Western Africa. J Clin Microbiol. 2015; 53:1156–1163. PMID: 25631805.4. Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009; 393:569–582. PMID: 18696055.5. Xia X, Xu Y, Zhao X, Li Q. Lateral flow immunoassay using europium chelate-loaded silica nanoparticles as labels. Clin Chem. 2009; 55:179–182. PMID: 18974359.6. Heiat M, Ranjbar R, Alavian SM. Classical and modern approaches used for viral hepatitis diagnosis. Hepat Mon. 2014; 14:e17632. PMID: 24829586.7. Chevaliez S, Poiteau L, Rosa I, Soulier A, Roudot-Thoraval F, Laperche S, et al. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin Microbiol Infect. 2016; 22:459.e1–459.e6. PMID: 26806260.8. Poiteau L, Soulier A, Rosa I, Roudot-Thoraval F, Hézode C, Pawlotsky JM, et al. Performance of rapid diagnostic tests for the detection of antibodies to hepatitis C virus in whole blood collected on dried blood spots. J Viral Hepat. 2016; 23:399–401. PMID: 26833561.9. Kim KR, Han YD, Chun HJ, Lee KW, Hong DK, Lee KN, et al. Encapsulation-stabilized, europium containing nanoparticle as a probe for time-resolved luminescence detection of cardiac troponin I. Biosensors (Basel). 2017; 7:E48. PMID: 29057816.10. Kim BC, Jeong JH, et al. Simplified laser fluorescence scanner for proteomics studies and early cancer diagnosis. In : Chance B, Chen M, editors. Photonics Asia-Optics in health care and biomedical optics: diagnostics and treatment. Shanghai: SPIE;2002. p. 103–108.11. Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016; 60:111–120. PMID: 27365041.12. Peeling RW, Mabey D. Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect. 2010; 16:1062–1069. PMID: 20670288.13. Smith BD, Jewett A, Drobeniuc J, Kamili S. Rapid diagnostic HCV antibody assays. Antivir Ther. 2012; 17:1409–1413. PMID: 23322678.14. Kim DS, Kim YT, Hong SB, Kim J, Huh NS, Lee MK, et al. Development of lateral flow assay based on size-controlled gold nanoparticles for detection of hepatitis B surface antigen. Sensors (Basel). 2016; 16:E2154. PMID: 27999291.15. Scheiblauer H, El-Nageh M, Diaz S, Nick S, Zeichhardt H, Grunert HP, et al. Performance evaluation of 70 hepatitis B virus (HBV) surface antigen (HBsAg) assays from around the world by a geographically diverse panel with an array of HBV genotypes and HBsAg subtypes. Vox Sang. 2010; 98:403–414. PMID: 20412171.16. Lin YH, Wang Y, Loua A, Day GJ, Qiu Y, Nadala EC Jr, et al. Evaluation of a new hepatitis B virus surface antigen rapid test with improved sensitivity. J Clin Microbiol. 2008; 46:3319–3324. PMID: 18701669.17. Bottero J, Boyd A, Gozlan J, Lemoine M, Carrat F, Collignon A, et al. Performance of rapid tests for detection of HBsAg and anti-HBsAb in a large cohort, France. J Hepatol. 2013; 58:473–478. PMID: 23183527.18. Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012; 55:1652–1661. PMID: 22213025.19. Hyun J, Ko DH, Kang HJ, Whang DH, Cha YJ, Kim HS. Evaluation of the VIDAS anti-HCV assay for detection of hepatitis C virus infection. Ann Lab Med. 2016; 36:550–554. PMID: 27578508.20. Park Y, Park JY, Kim MJ, Kim HS. Comparison of the diagnostic performance of Elecsys Anti-HCV II and Elecsys and Vitros Anti-HCV assays. J Lab Med Qual Assur. 2012; 34:51–56.21. Esteban JI, van Helden J, Alborino F, Bürgisser P, Cellerai C, Pantaleo G, et al. Multicenter evaluation of the Elecsys® anti-HCV II assay for the diagnosis of hepatitis C virus infection. J Med Virol. 2013; 85:1362–1368. PMID: 23765774.22. Kim S, Kim JH, Yoon S, Park YH, Kim HS. Clinical performance evaluation of four automated chemiluminescence immunoassays for hepatitis C virus antibody detection. J Clin Microbiol. 2008; 46:3919–3923. PMID: 18945839.23. Oh EJ, Chang J, Yang JY, Kim Y, Park YJ, Han K. Different signal-to-cut-off ratios from three automated anti-hepatitis C virus chemiluminescence immunoassays in relation to results of recombinant immunoblot assays and nucleic acid testing. Blood Transfus. 2013; 11:471–473. PMID: 23245710.24. Zacher BJ, Moriconi F, Bowden S, Hammond R, Louisirirotchanakul S, Phisalprapa P, et al. Multicenter evaluation of the Elecsys hepatitis B surface antigen quantitative assay. Clin Vaccine Immunol. 2011; 18:1943–1950. PMID: 21880853.25. Louisirirotchanakul S, Khupulsup K, Akraekthalin S, Chan KP, Saw S, Aw TC, et al. Comparison of the technical and clinical performance of the Elecsys HBsAg II assay with the Architect, AxSym, and Advia Centaur HBsAg screening assays. J Med Virol. 2010; 82:755–762. PMID: 20336717.26. Yoo SJ, Wang LL, Ning HC, Tao CM, Hirankarn N, Kuakarn S, et al. Evaluation of the Elecsys Anti-HCV II assay for routine hepatitis C virus screening of different Asian Pacific populations and detection of early infection. J Clin Virol. 2015; 64:20–27. PMID: 25728074.27. Popp C, Krams D, Beckert C, Buenning C, Queirós L, Piro L, et al. HBsAg blood screening and diagnosis: performance evaluation of the ARCHITECT HBsAg qualitative and ARCHITECT HBsAg qualitative confirmatory assays. Diagn Microbiol Infect Dis. 2011; 70:479–485. PMID: 21658874.28. Tuaillon E, Mondain AM, Nagot N, Ottomani L, Kania D, Nogue E, et al. Comparison of serum HBsAg quantitation by four immunoassays, and relationships of HBsAg level with HBV replication and HBV genotypes. PLoS One. 2012; 7:e32143. PMID: 22403628.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of 3 Automated Immunoassays for Hepatitis B Surface Antigen
- Prevalence of hepatitis B surface antigen(HBsAg), anti-hepatitis C virus(HCV) antibody-treponemal antibody and anti-HIV-1 antibody in donor's bloods
- A Study on Hepatitis B Surface Antigen in Tear of Hepatitis Patients
- A Study on Hepatitis B Surface Antigen in Tear of Hepatitis Patients
- Spontaneous Clearance of Hepatitis B Surface Antigenemia in a Hemodialysis Patient