Ann Dermatol.
2018 Dec;30(6):688-693. 10.5021/ad.2018.30.6.688.
A Pilot Study to Evaluate the Efficacy and Safety of Treatment with Botulinum Toxin in Patients with Recalcitrant and Persistent Erythematotelangiectatic Rosacea
- Affiliations
-
- 1Department of Dermatology, Chung-Ang University College of Medicine, Seoul, Korea. drseo@cau.ac.kr
- KMID: 2428924
- DOI: http://doi.org/10.5021/ad.2018.30.6.688
Abstract
- BACKGROUND
There are few pharmacologic options to reduce erythema and flushing in patients with recalcitrant erythematotelangiectatic rosacea (ETR). We previously reported two cases of refractory flushing and erythema of rosacea that were successfully treated with intradermal botulinum toxin injection, and additional research is needed to prove the efficacy and safety of this treatment.
OBJECTIVE
To report the efficacy and safety of botulinum toxin injection as an aid in persistent erythema of rosacea patients.
METHODS
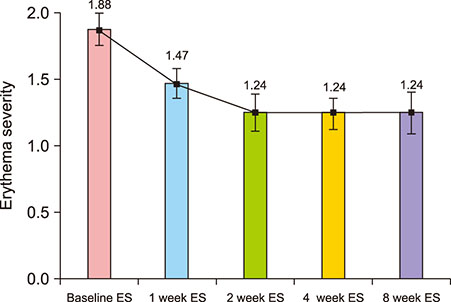
A total of 20 Korean patients with recalcitrant ETR were enrolled to receive treatment by injection of botulinum toxin. Patients received one treatment of intradermal botulinum toxin injection and were assessed 1, 2, 4, and 8 weeks after treatment. The severity of erythema and telangiectasia was investigated by a non-treating physician, and the Erythema Index (EI) was assessed by mexameter at each visit. Patient satisfaction and any adverse events were also assessed at each visit.
RESULTS
17 patients completed all follow-up visits and were included in the analysis. Intradermal injection of botulinum toxin significantly reduced erythema severity and EI in ETR patients. Patients reported a satisfaction score of 2.94±0.56 at 8 weeks after treatment. Except for three patients who discontinued the study early due to inconvenience of facial muscle paralysis, 17 patients participating in the final analysis did not report side effects except injection pain at the time of the procedure.
CONCLUSION
Intradermal injection of botulinum toxin can be used as an effective and relatively safe adjuvant agent for recalcitrant and persistent erythema of ETR patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Schwab VD, Sulk M, Seeliger S, Nowak P, Aubert J, Mess C, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011; 15:53–62.
Article2. Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012; 11:e76–e79.3. Bloom BS, Payongayong L, Mourin A, Goldberg DJ. Impact of intradermal abobotulinumtoxinA on facial erythema of rosacea. Dermatol Surg. 2015; 41:Suppl 1. S9–S16.
Article4. Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008; 7:847–850.5. Oh YJ, Lee NY, Suh DH, Koh JS, Lee SJ, Shin MK. A split-face study using botulinum toxin type B to decrease facial erythema index. J Cosmet Laser Ther. 2011; 13:243–248.
Article6. Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon. 2000; 38:245–258.
Article7. Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache. 2004; 44:35–42.
Article8. Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005; 174:977–982.9. Gessert CE, Bamford JT. Measuring the severity of rosacea: a review. Int J Dermatol. 2003; 42:444–448.
Article10. Park KY, Hyun MY, Jeong SY, Kim BJ, Kim MN, Hong CK. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015; 230:299–301.
Article11. Kellogg DL Jr, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, et al. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995; 77:1222–1228.
Article12. Kellogg DL Jr, Zhao JL, Wu Y, Johnson JM. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J Appl Physiol (1985). 2012; 113:1512–1518.
Article13. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013; 69:6 Suppl 1. S15–S26.
Article14. Steinhoff M, Buddenkotte J, Aubert J, Sulk M, Novak P, Schwab VD, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011; 15:2–11.
Article15. Drummond PD, Su D. Endothelial and axon reflex vasodilatation to acetylcholine in rosacea-affected skin. Arch Dermatol Res. 2012; 304:133–137.
Article16. Krämer HH, Angerer C, Erbguth F, Schmelz M, Birklein F. Botulinum toxin A reduces neurogenic flare but has almost no effect on pain and hyperalgesia in human skin. J Neurol. 2003; 250:188–193.
Article17. Tugnoli V, Capone JG, Eleopra R, Quatrale R, Sensi M, Gastaldo E, et al. Botulinum toxin type A reduces capsaicinevoked pain and neurogenic vasodilatation in human skin. Pain. 2007; 130:76–83.
Article18. Seeliger S, Buddenkotte J, Schmidt-Choudhury A, Rosignoli C, Shpacovitch V, von Arnim U, et al. Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo. Am J Pathol. 2010; 177:2563–2575.19. Goswami C, Rademacher N, Smalla KH, Kalscheuer V, Ropers HH, Gundelfinger ED, et al. TRPV1 acts as a synaptic protein and regulates vesicle recycling. J Cell Sci. 2010; 123:2045–2057.
Article20. Sulk M, Seeliger S, Aubert J, Schwab VD, Cevikbas F, Rivier M, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012; 132:1253–1262.
Article21. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004; 173:2909–2912.
Article22. Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, et al. Activation of TRPV1 mediates calcitonin generelated peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009; 29:4981–4992.
Article23. Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, et al. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis. 2012; 48:367–378.
Article24. Li X, Coffield JA. Structural and functional interactions between transient receptor potential vanilloid subfamily 1 and botulinum neurotoxin serotype A. PLos One. 2016; 11:e0143024.
Article25. Eshghi G, Khezrian L, Alirezaei P. Botulinum toxin A in treatment of facial flushing. Acta Med Iran. 2016; 54:454–457.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of severe erythematotelangiectatic rosacea with intense pulsed light: a case report
- Case report: treating vitiligo with intense pulsed light
- Novel Photopneumatic Therapy for the Treatment of Rosacea
- Clinical Application of Botulinum Toxin to Contact Granuloma and Vocal Nodule
- Application of Botulinum Toxin in Pain Management