Korean J Radiol.
2017 Apr;18(2):392-401. 10.3348/kjr.2017.18.2.392.
Concurrent Low Brain and High Liver Uptake on FDG PET Are Associated with Cardiovascular Risk Factors
- Affiliations
-
- 1Department of Nuclear Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon 51353, Korea.
- 2Department of Nuclear Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan 49267, Korea.
- 3Department of Nuclear Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan 49241, Korea. pnuhnm@gmail.com
- KMID: 2427952
- DOI: http://doi.org/10.3348/kjr.2017.18.2.392
Abstract
OBJECTIVE
Concurrent low brain and high liver uptake are sometimes observed on fluorine-18-labeled fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET). We investigated the potential clinical significance of this uptake pattern related to metabolic syndrome (MS).
MATERIALS AND METHODS
We retrospectively reviewed data from 264 consecutive males who had undergone general health check-ups, including FDG PET/CT scans. After an overnight fast, the men had their peripheral blood drawn and the levels of various laboratory parameters measured; an FDG PET/CT scan was performed on the same day. We measured the maximum standardized uptake values of the brain and liver from regions of interest manually placed over the frontal cortex at the level of the centrum semiovale and the right lobe of the liver parenchyma, respectively.
RESULTS
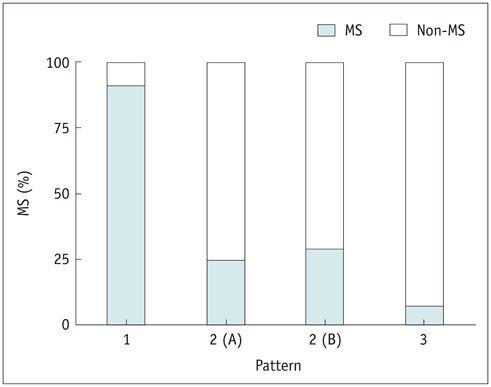
Fasting blood glucose (FBG; odds ratio [OR] = 1.063, p < 0.001) and glycated hemoglobin (HbA1c; OR = 3.634, p = 0.010) were the strongest predictive factors for low brain FDG uptake, whereas waist circumference (OR = 1.200, p < 0.001) and γ-glutamyl transpeptidase (OR = 1.012, p = 0.001) were the strongest predictive factors for high liver uptake. Eleven subjects (4.2%) showed concurrent low brain and high liver FDG uptake, and all but one of these subjects (90.9%) had MS. Systolic blood pressure, waist circumference, FBG, triglyceride, alanine aminotransferase, insulin resistance (measured by homeostasis model assessment), insulin, HbA1c, and body mass index were higher in subjects with this FDG uptake pattern than in those without (all, p < 0.001).
CONCLUSION
Concurrent low brain and high liver FDG uptake were closely associated with MS. Moreover, subjects with this pattern had higher values for various cardiovascular risk factors than did those without.
Keyword
MeSH Terms
-
Adult
Aged
Blood Glucose/analysis
Brain/*metabolism
Cardiovascular Diseases/diagnosis/etiology
Fluorodeoxyglucose F18/chemistry
Glycated Hemoglobin A/analysis
Humans
Liver/*metabolism
Logistic Models
Male
Metabolic Syndrome/complications/pathology
Middle Aged
Odds Ratio
Positron Emission Tomography Computed Tomography
Positron-Emission Tomography
Radiopharmaceuticals/chemistry/*metabolism
Retrospective Studies
Risk Factors
Waist Circumference
gamma-Glutamyltransferase/analysis
Blood Glucose
Glycated Hemoglobin A
Radiopharmaceuticals
Fluorodeoxyglucose F18
gamma-Glutamyltransferase
Figure
Cited by 1 articles
-
Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991; 32:623–648. discussion 649-650.2. Büsing KA, Schönberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol. 2013; 40:206–213.3. Minamimoto R, Takahashi N, Inoue T. FDG-PET of patients with suspected renal failure: standardized uptake values in normal tissues. Ann Nucl Med. 2007; 21:217–222.4. Langbaum JB, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol Aging. 2012; 33:827.e11–827.e19.5. Reiman EM, Chen K, Langbaum JB, Lee W, Reschke C, Bandy D, et al. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer's disease and normal aging. Neuroimage. 2010; 49:169–176.6. Miao Q, Zhang S, Guan YH, Ye HY, Zhang ZY, Zhang QY, et al. Reversible changes in brain glucose metabolism following thyroid function normalization in hyperthyroidism. AJNR Am J Neuroradiol. 2011; 32:1034–1042.7. Loessner A, Alavi A, Lewandrowski KU, Mozley D, Souder E, Gur RE. Regional cerebral function determined by FDG-PET in healthy volunteers: normal patterns and changes with age. J Nucl Med. 1995; 36:1141–1149.8. Petit-Taboué MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998; 7:176–184.9. Ivançevic’ V, Alavi A, Souder E, Mozley PD, Gur RE, Bénard F, et al. Regional cerebral glucose metabolism in healthy volunteers determined by fluordeoxyglucose positron emission tomography: appearance and variance in the transaxial, coronal, and sagittal planes. Clin Nucl Med. 2000; 25:596–602.10. Liu G, Li Y, Hu P, Cheng D, Shi H. The combined effects of serum lipids, BMI, and fatty liver on 18F-FDG uptake in the liver in a large population from China: an 18F-FDG-PET/CT study. Nucl Med Commun. 2015; 36:709–716.11. Kuruva M, Mittal BR, Abrar ML, Kashyap R, Bhattacharya A. Multivariate analysis of various factors affecting background liver and mediastinal standardized uptake values. Indian J Nucl Med. 2012; 27:20–23.12. Kubota K, Watanabe H, Murata Y, Yukihiro M, Ito K, Morooka M, et al. Effects of blood glucose level on FDG uptake by liver: a FDG-PET/CT study. Nucl Med Biol. 2011; 38:347–351.13. Kamimura K, Nagamachi S, Wakamatsu H, Higashi R, Ogita M, Ueno S, et al. Associations between liver (18)F fluoro-2-deoxy-D-glucose accumulation and various clinical parameters in a Japanese population: influence of the metabolic syndrome. Ann Nucl Med. 2010; 24:157–161.14. Lin CY, Ding HJ, Lin T, Lin CC, Kuo TH, Kao CH. Positive correlation between serum liver enzyme levels and standard uptake values of liver on FDG-PET. Clin Imaging. 2010; 34:109–112.15. Lin CY, Ding HJ, Lin CC, Chen CC, Sun SS, Kao CH. Impact of age on FDG uptake in the liver on PET scan. Clin Imaging. 2010; 34:348–350.16. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–2752.17. Roy FN, Beaulieu S, Boucher L, Bourdeau I, Cohade C. Impact of intravenous insulin on 18F-FDG PET in diabetic cancer patients. J Nucl Med. 2009; 50:178–183.18. Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, et al. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology. 1995; 195:47–52.19. Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010; 195:310–320.20. Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn study. Circulation. 2005; 112:666–673.21. Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010; 2:180–193.22. Crippa F, Gavazzi C, Bozzetti F, Chiesa C, Pascali C, Bogni A, et al. The influence of blood glucose levels on [18F]fluorodeoxyglucose (FDG) uptake in cancer: a PET study in liver metastases from colorectal carcinomas. Tumori. 1997; 83:748–752.23. Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med. 1993; 34:1–6.24. Cheung JY, Conover C, Regen DM, Whitfield CF, Morgan HE. Effect of insulin on kinetics of sugar transport in heart muscle. Am J Physiol. 1978; 234:E70–E78.25. Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC 3rd. Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994; 89:793–798.26. Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007; 50:2239–2244.27. Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012; 122:1316–1338.28. Messier C, Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer's disease. Neural Plast. 2005; 12:311–328.29. Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: implication for Alzheimer's disease. Am J Pathol. 2009; 175:2089–2098.30. Smith SR, Ravussin E. Emerging paradigms for understanding fatness and diabetes risk. Curr Diab Rep. 2002; 2:223–230.31. Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992; 33:1972–1980.32. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115:1111–1119.33. Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008; 14:185–192.34. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001; 50:1844–1850.35. Lee DH, Jacobs DR Jr. Association between serum gamma-glutamyltransferase and C-reactive protein. Atherosclerosis. 2005; 178:327–330.36. Kang YH, Min HK, Son SM, Kim IJ, Kim YK. The association of serum gamma glutamyltransferase with components of the metabolic syndrome in the Korean adults. Diabetes Res Clin Pract. 2007; 77:306–313.37. Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993; 189:847–850.38. Lindholm H, Johansson O, Jonsson C, Jacobsson H. The distribution of FDG at PET examinations constitutes a relative mechanism: significant effects at activity quantification in patients with a high muscular uptake. Eur J Nucl Med Mol Imaging. 2012; 39:1685–1690.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Esophageal Leiomyoma Showing High FDG Uptake on F-18 FDG PET
- High FDG Uptake in Sclerosing Hemangioma
- Correlation of Hepatic 18F-Fluorodeoxyglucose Uptake with Fatty Liver
- A Case of Hepatic Angiomyolipoma Showing Different Uptake on F-18 FDG and C-11 Acetate PET
- Carotid Artery FDG Uptake May Serve as a Biomarker for Cardiovascular Risk Stratification in Asymptomatic Adults