Allergy Asthma Immunol Res.
2019 Jan;11(1):116-128. 10.4168/aair.2019.11.1.116.
Mugwort Pollen-Related Food Allergy: Lipid Transfer Protein Sensitization and Correlation With the Severity of Allergic Reactions in a Chinese Population
- Affiliations
-
- 1Department of Allergy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China. doctoryinjia@163.com
- 2Beijing Key Laboratory of Precision Medicine for Diagnosis and Treatment on Allergic Diseases, Beijing, China.
- KMID: 2426790
- DOI: http://doi.org/10.4168/aair.2019.11.1.116
Abstract
- PURPOSE
Little is known about the importance of lipid transfer protein (LTP) sensitization in China. In this study, we investigated the relationship between LTP sensitization and the severity of clinical symptoms in a population of patients with mugwort pollen-related food allergy.
METHODS
Food-induced symptoms were evaluated in 148 patients with mugwort pollen allergy by a standardized questionnaire. Specific immunoglobulin E (IgE) to Art v 1, Art v 3, Pru p 3, Ara h 9 and Cor a 8 were quantified by ImmunoCAP. Immunoblotting of peach extracts were performed with sera from peach-allergic patients.
RESULTS
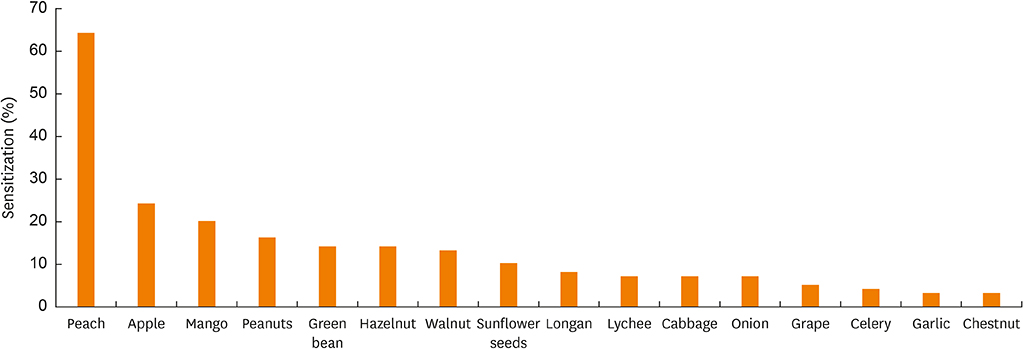
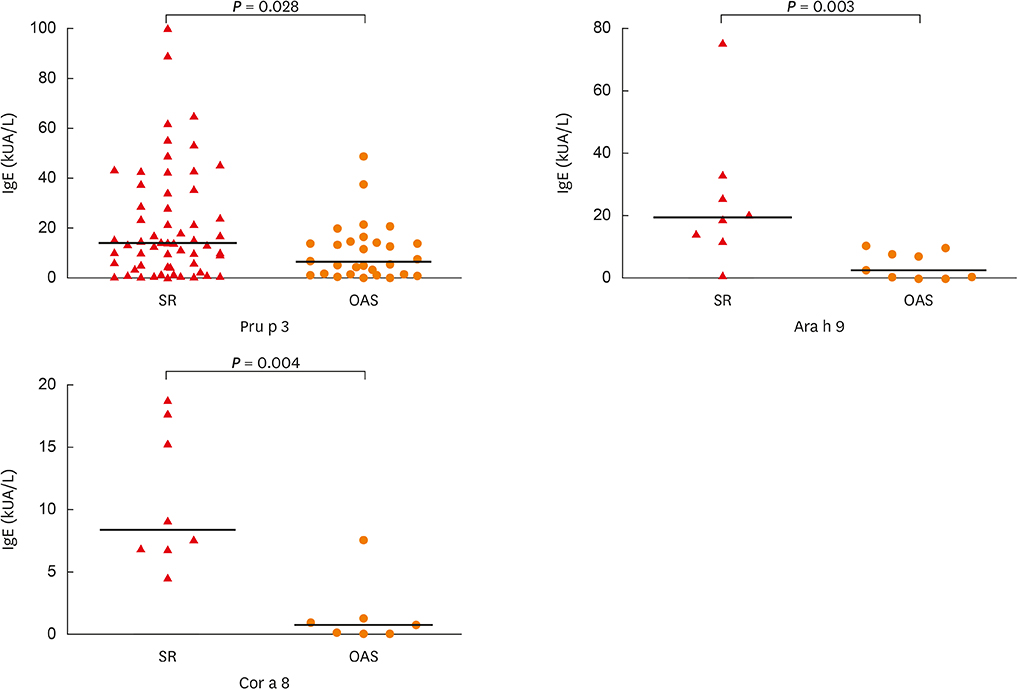
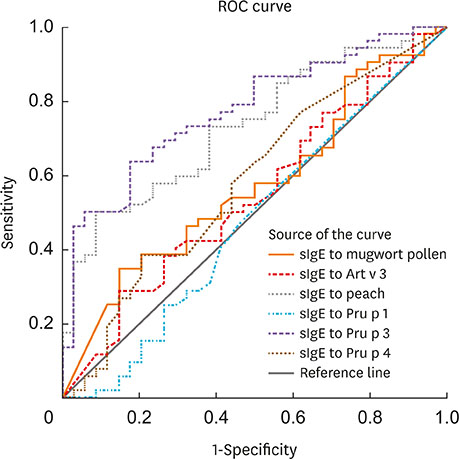
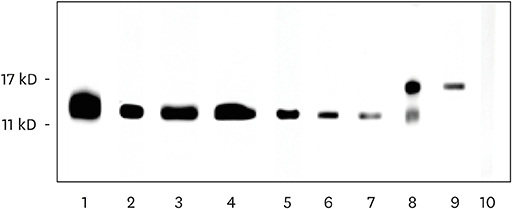
In total, 72% (107/148) of the study population experienced food allergy. Forty-eight percent (51/107) of patients with mugwort pollen-related food allergy experienced at least 1 episode of food-induced anaphylaxis. Food allergy correlated with IgE reactivity to Art v 3, but not to Art v 1. Sensitization to Pru p 3, Ara h 9 or Cor a 8 was prevalent (80%, 69 or 63%, respectively) among individuals with food allergy. Food allergic patients with systemic reactions (SR) had higher values for Pru p 3, Ara h 9 and Cor a 8 than patients with oral allergy syndrome (OAS). Furthermore, the strong IgE reactivity detected in immunoblots of peach extracts indicated that Pru p 3 was the major allergen and was more prevalent in patients with SR than in patients with OAS (100% vs. 55%).
CONCLUSIONS
LTPs are major food allergens for mugwort pollen-related food allergy in China, and may contribute to SR.
MeSH Terms
Figure
Cited by 1 articles
-
Revised Pollen Calendar in Korea
Jung-Won Park
Allergy Asthma Immunol Res. 2020;12(2):171-172. doi: 10.4168/aair.2020.12.2.171.
Reference
-
1. Ye S, Zhang J, Gu R. Investigation of aeroborne allergenic pollens in different regions of China. 1st edition. Beijing: Beijing Publishers;1991.2. Popescu FD. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015; 5:31–50.
Article3. Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010; 104:101–108.
Article4. Ballmer-Weber BK, Hoffmann A, Wüthrich B, Lüttkopf D, Pompei C, Wangorsch A, et al. Influence of food processing on the allergenicity of celery: DBPCFC with celery spice and cooked celery in patients with celery allergy. Allergy. 2002; 57:228–235.
Article5. Figueroa J, Blanco C, Dumpiérrez AG, Almeida L, Ortega N, Castillo R, et al. Mustard allergy confirmed by double-blind placebo-controlled food challenges: clinical features and cross-reactivity with mugwort pollen and plant-derived foods. Allergy. 2005; 60:48–55.
Article6. Pastorello EA, Pravettoni V, Farioli L, Rivolta F, Conti A, Ispano M, et al. Hypersensitivity to mugwort (Artemisia vulgaris) in patients with peach allergy is due to a common lipid transfer protein allergen and is often without clinical expression. J Allergy Clin Immunol. 2002; 110:310–317.
Article7. Wen Z, Ye S. A report of 50 patients with artemisia pollenosis and plant food allergy. Zhonghua Yi Xue Za Zhi. 2002; 82:626–629.8. Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006; 61:461–476.
Article9. Ma S, Yin J, Jiang N. Component-resolved diagnosis of peach allergy and its relationship with prevalent allergenic pollens in China. J Allergy Clin Immunol. 2013; 132:764–767.
Article10. Fernández-Rivas M, Bolhaar S, González-Mancebo E, Asero R, van Leeuwen A, Bohle B, et al. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006; 118:481–488.
Article11. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006; 47:373–380.
Article12. Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012; 67:18–24.13. Pastorello EA, Farioli L, Pravettoni V, Ortolani C, Ispano M, Monza M, et al. The major allergen of peach (Prunus persica) is a lipid transfer protein. J Allergy Clin Immunol. 1999; 103:520–526.
Article14. Flores E, Cervera L, Sanz ML, Diaz-Perales A, Fernández J. Plant food allergy in patients with pollinosis from the Mediterranean area. Int Arch Allergy Immunol. 2012; 159:346–354.
Article15. Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, Greiderer A, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011; 127:616–622.e1.
Article16. Skypala IJ, Calderon MA, Leeds AR, Emery P, Till SJ, Durham SR. Development and validation of a structured questionnaire for the diagnosis of oral allergy syndrome in subjects with seasonal allergic rhinitis during the UK birch pollen season. Clin Exp Allergy. 2011; 41:1001–1011.
Article17. Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, Knulst AC, et al. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015; 70:1079–1090.
Article18. Gruber P, Gadermaier G, Bauer R, Weiss R, Wagner S, Leonard R, et al. Role of the polypeptide backbone and post-translational modifications in cross-reactivity of Art v 1, the major mugwort pollen allergen. Biol Chem. 2009; 390:445–451.
Article19. Gao ZS, Yang ZW, Wu SD, Wang HY, Liu ML, Mao WL, et al. Peach allergy in China: a dominant role for mugwort pollen lipid transfer protein as a primary sensitizer. J Allergy Clin Immunol. 2013; 131:224–226.e1-3.20. Lombardero M, García-Sellés FJ, Polo F, Jimeno L, Chamorro MJ, García-Casado G, et al. Prevalence of sensitization to Artemisia allergens Art v 1, Art v 3 and Art v 60 kDa. Cross-reactivity among Art v 3 and other relevant lipid-transfer protein allergens. Clin Exp Allergy. 2004; 34:1415–1421.
Article21. García-Sellés FJ, Díaz-Perales A, Sánchez-Monge R, Alcántara M, Lombardero M, Barber D, et al. Patterns of reactivity to lipid transfer proteins of plant foods and Artemisia pollen: an in vivo study. Int Arch Allergy Immunol. 2002; 128:115–122.22. Palacín A, Cumplido J, Figueroa J, Ahrazem O, Sánchez-Monge R, Carrillo T, et al. Cabbage lipid transfer protein Bra o 3 is a major allergen responsible for cross-reactivity between plant foods and pollens. J Allergy Clin Immunol. 2006; 117:1423–1429.23. Mothes-Luksch N, Raith M, Stingl G, Focke-Tejkl M, Razzazi-Fazeli E, Zieglmayer R, et al. Pru p 3, a marker allergen for lipid transfer protein sensitization also in Central Europe. Allergy. 2017; 72:1415–1418.
Article24. Scala E, Till SJ, Asero R, Abeni D, Guerra EC, Pirrotta L, et al. Lipid transfer protein sensitization: reactivity profiles and clinical risk assessment in an Italian cohort. Allergy. 2015; 70:933–943.
Article25. Tolkki L, Alanko K, Petman L, Skydtsgaard MB, Milvang PG, Seppälä U, et al. Clinical characterization and IgE profiling of birch (Betula verrucosa)--allergic individuals suffering from allergic reactions to raw fruits and vegetables. J Allergy Clin Immunol Pract. 2013; 1:623–631.e1.
Article26. Datema MR, Zuidmeer-Jongejan L, Asero R, Barreales L, Belohlavkova S, de Blay F, et al. Hazelnut allergy across Europe dissected molecularly: A EuroPrevall outpatient clinic survey. J Allergy Clin Immunol. 2015; 136:382–391.27. Pascal M, Muñoz-Cano R, Reina Z, Palacín A, Vilella R, Picado C, et al. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012; 42:1529–1539.
Article28. Yin J, Yue FM, Wang LL, He HJ, Xu T, Zhang HY, et al. The clinical study of the relationship between allergic rhinitis and allergic asthma in the patients with autumnal pollinosis. Zhonghua Yi Xue Za Zhi. 2005; 85:1683–1687.29. Yin J, Yue FM, Wang LL, He HJ, Xu T, Li H, et al. Natural course from rhinitis to asthma in the patients with autumnal pollinosis: a clinical study of 1096 patients. Zhonghua Yi Xue Za Zhi. 2006; 86:1628–1632.30. Uasuf CG, Villalta D, Conte ME, Di Sano C, Barrale M, Cantisano V, et al. Different co-sensitizations could determine different risk assessment in peach allergy? Evaluation of an anaphylactic biomarker in Pru p 3 positive patients. Clin Mol Allergy. 2015; 13:30.
Article31. Le TM, van Hoffen E, Lebens AF, Bruijnzeel-Koomen CA, Knulst AC. Anaphylactic versus mild reactions to hazelnut and apple in a birch-endemic area: different sensitization profiles? Int Arch Allergy Immunol. 2013; 160:56–62.
Article32. Scheurer S, Lauer I, Foetisch K, San Miguel Moncin M, Retzek M, Hartz C, et al. Strong allergenicity of Pru av 3, the lipid transfer protein from cherry, is related to high stability against thermal processing and digestion. J Allergy Clin Immunol. 2004; 114:900–907.
Article33. Offermann LR, Schlachter CR, Perdue ML, Majorek KA, He JZ, Booth WT, et al. Structural, functional, and immunological characterization of profilin panallergens Amb a 8, Art v 4, and Bet v 2. J Biol Chem. 2016; 291:15447–15459.
Article34. Ganglberger E, Radauer C, Grimm R, Hoffmann-Sommergruber K, Breiteneder H, Scheiner O, et al. N-terminal sequences of high molecular weight allergens from celery tuber. Clin Exp Allergy. 2000; 30:566–570.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bee pollen-induced anaphylaxis: Report of a patient with oral allergy syndrome
- Pollen-food allergy syndrome in children
- Oral allergy syndrome
- Relationship between Sensitization to Outdoor Aeroallergen and Month of Birth
- Are there any links between mugwort pollen and food allergens such as celery and carrot based upon allergy skin prick tests?