J Korean Orthop Assoc.
1996 Apr;31(2):388-394. 10.4055/jkoa.1996.31.2.388.
Hydrogen Peroxide Production in Neutrophils after Tourniquet Release
- KMID: 2423620
- DOI: http://doi.org/10.4055/jkoa.1996.31.2.388
Abstract
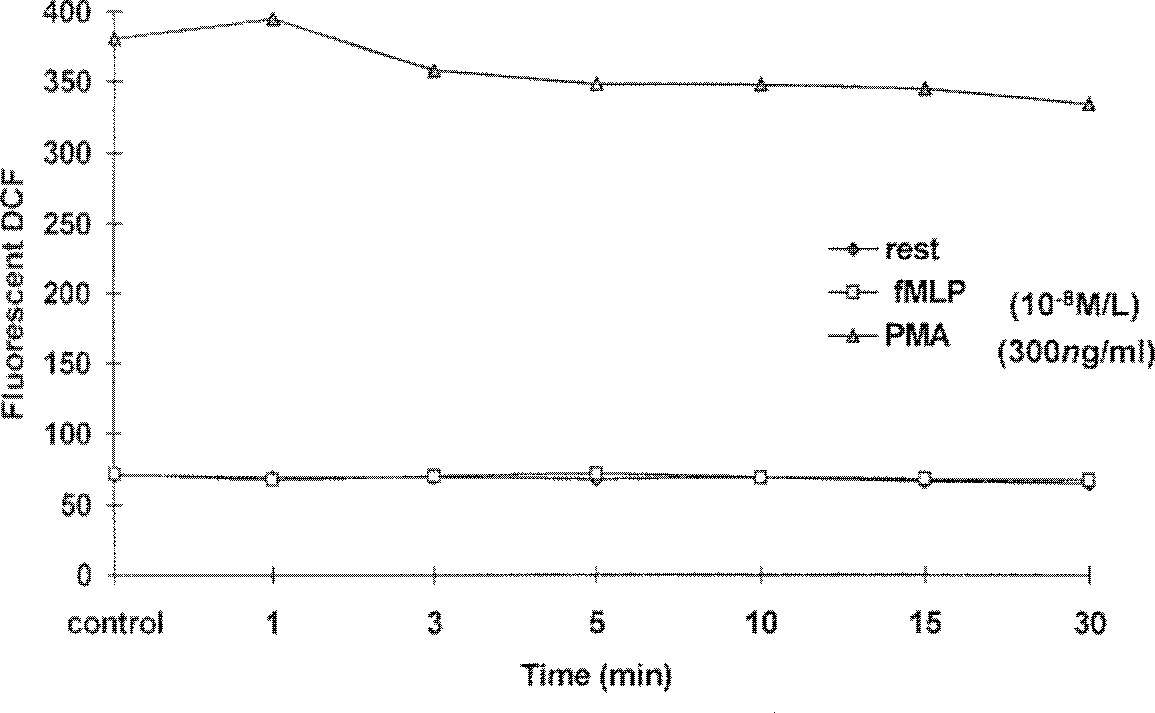
- The use of lower extremity tourniquets for procedures of the lower leg is considered routine in orthopedic surgery, but, lower extremity tourniquets do harm occasionally. While the tourniquet is inflated, metabolic changes such as increased PaCO2 , lactic acid, and serum potassium and decreased level of PaO2 and pH occur in the ischemic limb. Deflation of tourniquet results in release of anaerobic metabolic products during ischemia into systemic circulation. In this ischemia/reperfusion situation, oxygen free radicals could potentially be produced during the reperfusion period by several mechanisms. One of these mechanisms is release of intracellular superoxide or hydrogen peroxide by activated neutrophils in the area. These reactive oxygen species(ROS) could be a causative factor for the postreperfusion no-flow, lung injury, induction of tourniquet shock, etc. The purpose of this clinical study was to investigate the effect of tourniquet deflation on the hemodynamic changes, changes of blood gas analysis, and hydrogen peroxide production using flow cytometric analysis of fluorescent DCF(Dichlorofluorescein). Quantitative analysis of fluorescent DCF was performed in resting and fMLP(N-formyl-methyonyl-leucyl-phenylalanine) or PMA(phorbol myristate acetate) stimulated neutrophils. Also differences of these factors between two groups of tourniquet time, one is less than one hour and the other more than one to two hours, were analysed. The hemodynamics(blood pressure, pulse rate), arterial PO2, bicarbonate, base excess, and hydrogen peroxide production showed no significant change before and after tourniquet release(p>0.05). Arterial pH and PaCO2 decreased significantly until 10 and 5 minutes after tourniquet release, respectively(p>0.05). Tourniquet time didn't reveal any significances differences. These results indicate that tourniquet application with400mmHg pressure and less than 2 hours does not release significant hydrogen peroxide into systemic circulation during reperfusion period after tourniquet release.
Keyword
MeSH Terms
-
Blood Gas Analysis
Blood Pressure
Clinical Study
Extremities
Flow Cytometry
Free Radicals
Hemodynamics
Hydrogen Peroxide*
Hydrogen*
Hydrogen-Ion Concentration
Ischemia
Lactic Acid
Leg
Lower Extremity
Lung Injury
Myristic Acid
Neutrophils*
Orthopedics
Oxygen
Potassium
Reperfusion
Shock
Superoxides
Tourniquets*
Free Radicals
Hydrogen
Hydrogen Peroxide
Lactic Acid
Myristic Acid
Oxygen
Potassium
Superoxides
Figure
Reference
-
1. Rang HJ, Han CD, Jahng JS, Ko So. Blood gas and electrolyte changes after tourniquet application in total knee replacement surgery. Yonsei Med J. 1992; 32:153–8.
Article2. Babior BM. Oxygen-dependent microbial killing by phagocytes. N Engl J Med,. 1978; 298:659–68.
Article3. Bass DA, Parce JW, DeChatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils:a graded response to membrane stimulation. J. Immunol. 1983; 130:1910–7.4. Bolton CF, Mcfarlane RM. Human pneumatic tourniquet paralysis. Neurology. 1978; 28:789–93.
Article5. Bucana C, Saiki l, Nayer R. Uptake and accumulation of the vital dye hydrocthidine in neoplastic cells. J Histochem Cytochem. 1986; 34:1109–13.6. De Chatelet LR. Inhibition of the respiratory burst in human polymorphonuclear neutrophils, a clinical review. J Reiiculoenlholhel Soc. 1978; 24:73–7.7. Eckhoff NL, Lond MS. Eng FRCS : Tourniquet paralysis. Lancet, 2:343-5, 1931.8). 1986; Forman HJ Thomas MJ;Oxidant production and bactericidal activity of phagocytes Ann Rev Physiol: 48:669–80.9. Kaufman RD, Walts LF. Tourniquet-induced hypertension. Br J Anaesth. 1982; 54:333–6.
Article10. Keston AS, Brandt R. The flurometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem,. 11:1–5. 1965.11. Moldaver J. Tourniquet paralysis syndrome. Arch Surg. 68:136–44. 1954.
Article12. Rossi F, Dri P, Bellavite P, Zabucchi G, Berton G. Oxidative metabolism of inflammatory cells. Adv inflammation Res. 1979; 1:139–44.13. Rudge P. Tourniquet paralysis with prolonged conduction block. J Bone Joint Surg. 56B:1974; 716–20.
Article14. Simonson SG, Zhang J, Canada AT, Su YF, Benveniste H, Piantadosi CA. Hydrogen peroxide production by monoamone oxidase during ischemia-reperfusion in the rat brain. J of Cerebral blood flow and metabolism,. 1993; 13:125–134.15. Solonen KA, Tarkkanen L, Narvanen S, Gordin R. Metabolic changes in the upper limb during tourniquet ischemia. Acta orthop Scand. 39:1968; 20–32.16. Thomas G, Roques B. Proton magnetic resonance studies of ehtidium bromide and its sodium borohy-dride reduced derivatives. FEBS Lett. 1972; 26:169–73.17. Wilgis EFS. Observations on the effects of tourniquet ischemia. J Bone Joint Surg. 53A:1343–5. 1971.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hydrogen Peroxide Production in Neutrophil after Tourniquet Release
- Neutrophil Apoptosis and H2O2 Release by LPS in Diabetics
- A case of hydrogen peroxide-induced proctocolitis in a child
- Longitudinal flowcytometric measurement of respiratory burstactivity of neutrophils in patients with pneumonia
- Decreased Hydrogen Peroxide Generation by Neutrophils from Acne Patients Treated with Isotretinoin