Lab Med Online.
2018 Oct;8(4):140-147. 10.3343/lmo.2018.8.4.140.
Clinical Usefulness of ImmuneCheckâ„¢ IgG for Rapid Semiquantitation of Total IgG
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. PARKJW@yuhs.ac
- 2Institute of Allergy, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Hanshin Medipia, Seoul, Korea.
- 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2421004
- DOI: http://doi.org/10.3343/lmo.2018.8.4.140
Abstract
- BACKGROUND
Conventional IgG assays require costly equipment and skilled experts. Semiquantitative measurement of total IgG using point-of-care testing devices may be the solution for these limitations. This study evaluated the reproducibility of the ImmuneCheckâ„¢ IgG assay (ProteomeTech Inc., Korea) and the correlation of its results with conventional laboratory IgG results in the serum and whole blood.
METHODS
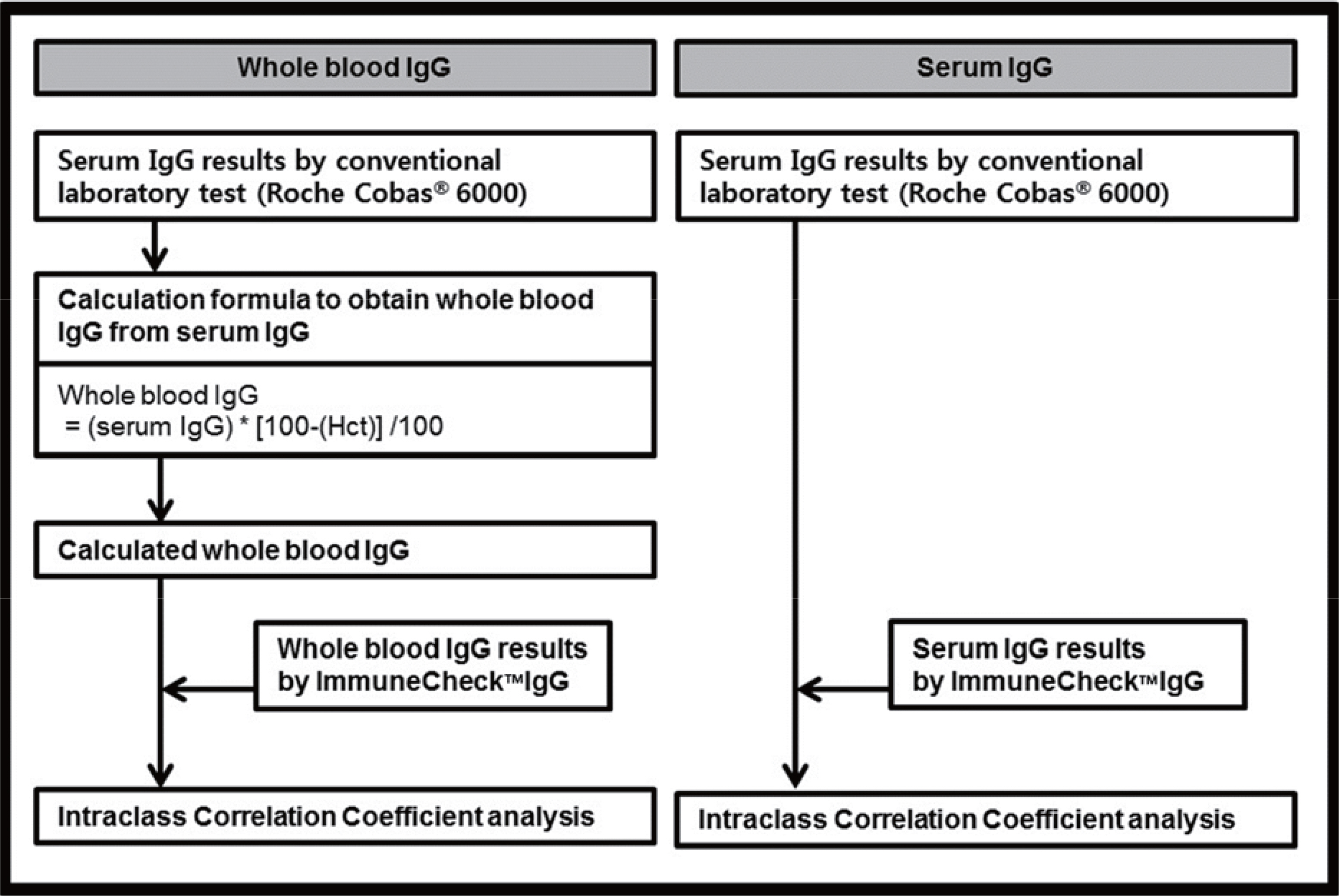
Both the serum and whole blood samples from 120 patients were used. To evaluate the intra-test reproducibility and inter-test correlation, intraclass correlation coefficient (ICC) analysis was used.
RESULTS
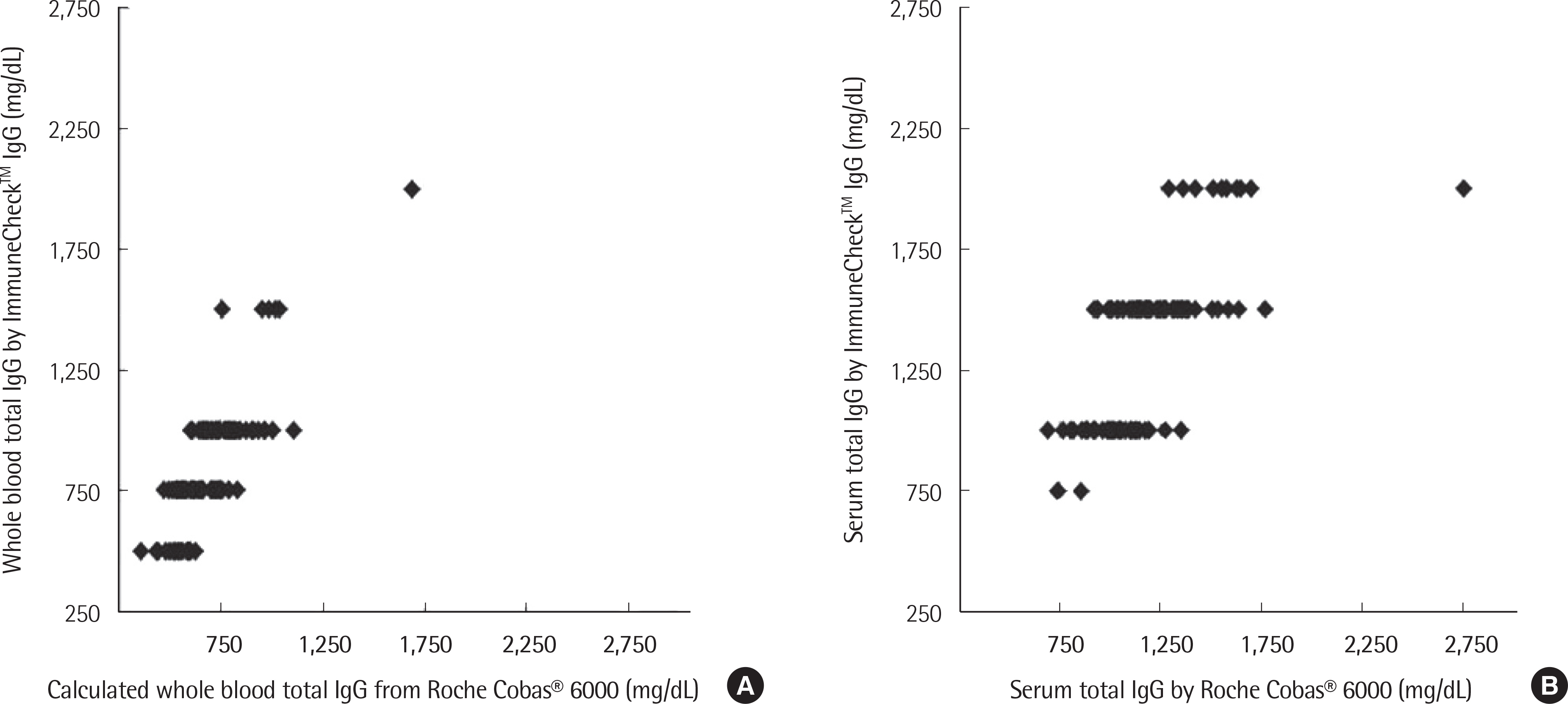
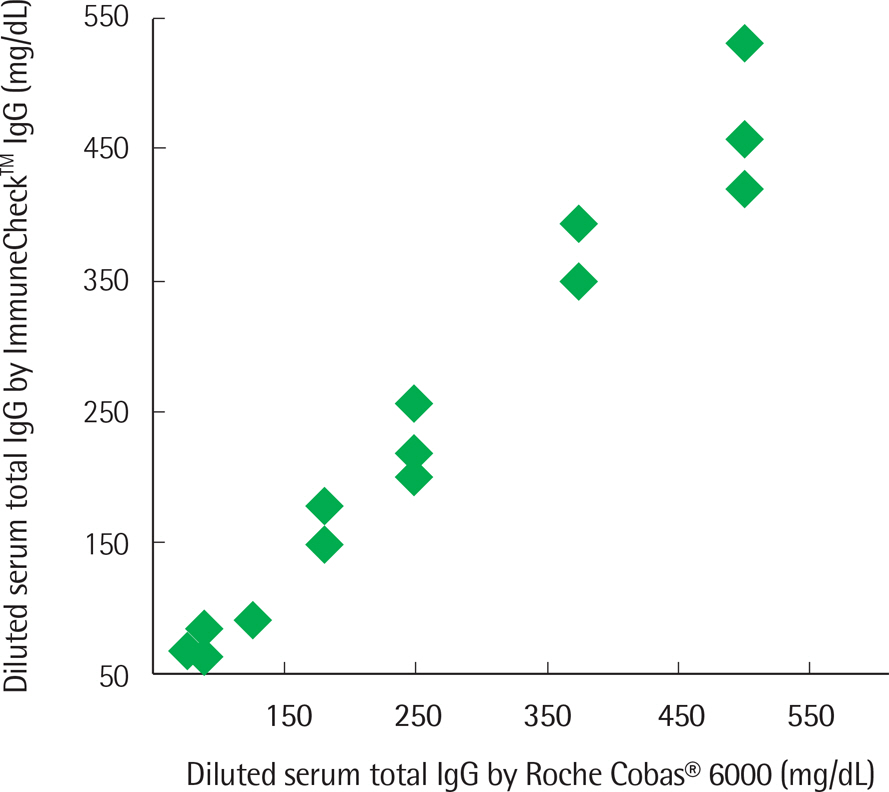
The concentration of serum total IgG measured by cobas® 6000 (Roche Diagnostics, Switzland) ranged from 690.4 to 2,756.4 mg/dL. The intra-test reproducibility of ImmuneCheckâ„¢ IgG was high (Serum ICC=0.724, P < 0.001; Whole blood ICC=0.843, P < 0.001). The inter-test correlation between the ImmuneCheckâ„¢ IgG and cobas® 6000 results was very good (Serum ICC=0.805, P < 0.001; Whole blood ICC=0.842, P < 0.001). Because there were no samples with a total IgG level lower than 600 mg/dL, the pre-existing serum samples were diluted and then the linearity tests were conducted. The intra-test reproducibility for the diluted serum samples was almost perfect (ICC=0.995, P < 0.001), and the inter-test correlation between the ImmuneCheckâ„¢ IgG and cobas® 6000 results was also strong (ICC=0.992, P < 0.001).
CONCLUSIONS
The ImmuneCheckâ„¢ IgG assay is reproducible and highly correlated with the conventional IgG assay for the serum and whole blood. It could be applied for the rapid detection of total IgG.
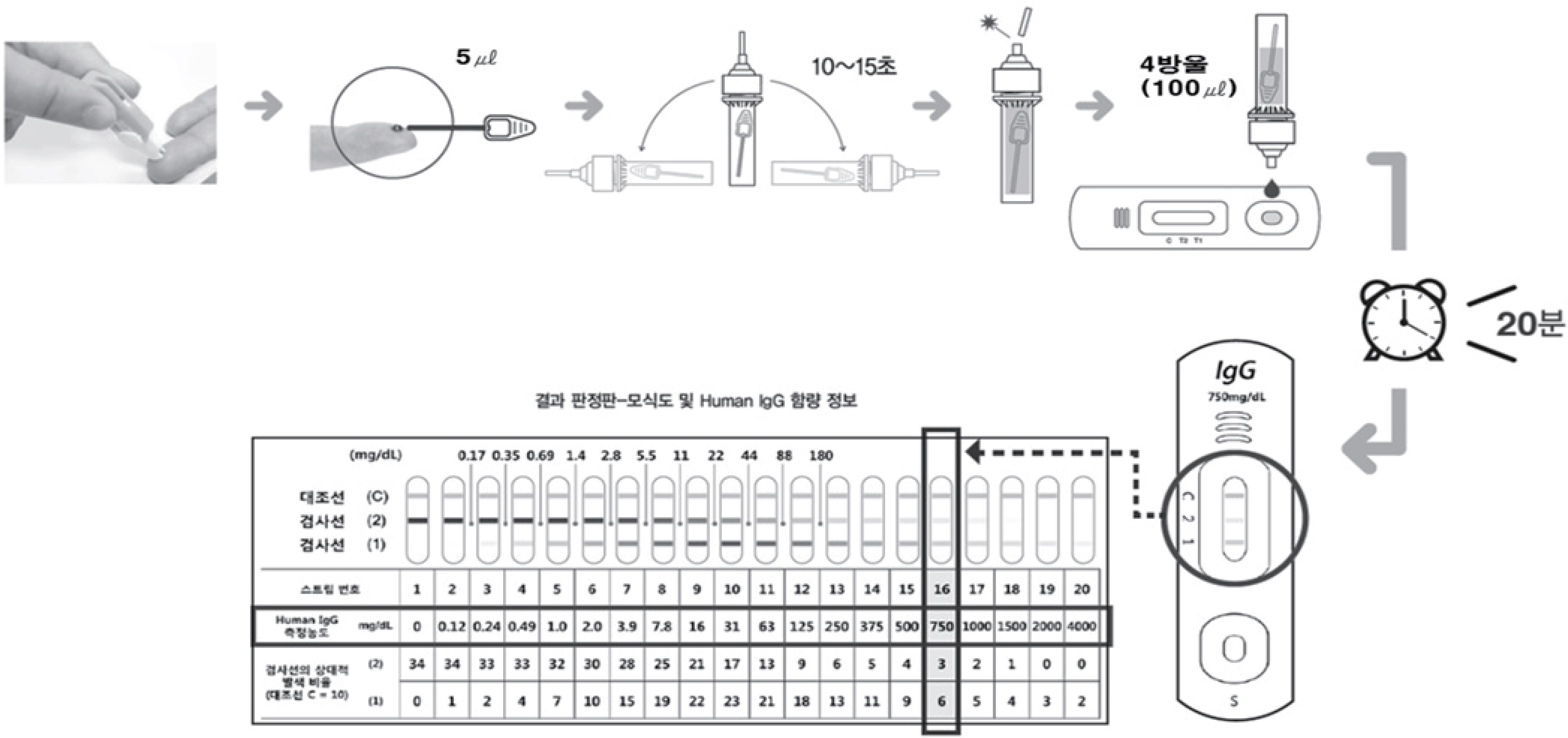
Figure
Reference
-
1.Schroeder HW Jr., Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010. 125:S41–52.
Article2.Aalberse R. The role of IgG antibodies in allergy and immunotherapy. Allergy. 2011. 66(Suppl 95):28–30.
Article3.Williams JW., Tjota MY., Sperling AI. The contribution of allergen-specifc IgG to the development of th2-mediated airway infammation. J Allergy (Cairo). 2012. 2012:236075.4.Meulenbroek LA., de Jong RJ., den Hartog Jager CF., Monsuur HN., Wouters D., Nauta AJ, et al. IgG antibodies in food allergy infuence allergen-antibody complex formation and binding to B cells: a role for complement receptors. J Immunol. 2013. 191:3526–33.5.Carmi Y., Spitzer MH., Linde IL., Burt BM., Prestwood TR., Perlman N, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015. 521:99–104.
Article6.Niu N., Zhang J., Huang T., Sun Y., Chen Z., Yi W, et al. IgG expression in human colorectal cancer and its relationship to cancer cell behaviors. PLoS One. 2012. 7:e47362.
Article7.Jefferis R., Kumararatne DS. Selective IgG subclass defciency: quan-tifcation and clinical relevance. Clin Exp Immunol. 1990. 81:357–67.8.Gonzalez-Quintela A., Alende R., Gude F., Campos J., Rey J., Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008. 151:42–50.
Article9.Puissant-Lubrano B., Peres M., Apoil PA., Congy-Jolivet N., Roubinet F., Blancher A. Immunoglobulin IgA, IgD, IgG, IgM and IgG subclass reference values in adults. Clin Chem Lab Med. 2015. 53:e359–61.
Article10.Wood RA., Lederman HM. Immunoglobulin defciency and recurrent upper respiratory tract infections. JAMA. 1987. 257:486.11.Slatter MA., Gennery AR. Clinical immunology review series: an approach to the patient with recurrent infections in childhood. Clin Exp Immunol. 2008. 152:389–96.
Article12.Raje N., Dinakar C. Overview of immunodefciency disorders. Immunol Allergy Clin North Am. 2015. 35:599–623.13.Supak Smolcic V., Bilic-Zulle L., Fisic E. Validation of methods performance for routine biochemistry analytes at Cobas 6000 analyzer series module c501. Biochem Med (Zagreb). 2011. 21:182–90.
Article14.Landis JR., Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–74.
Article15.Bonagura VR., Marchlewski R., Cox A., Rosenthal DW. Biologic IgG level in primary immunodefciency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008. 122:210–2.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum IgG and IgG subclass in bronchial asthma
- Impact of high IgG titer in ABO-incompatible kidney transplant
- Intracranial synthesis of specific IgG antibody in cerebrospinal fluid of neurocysticercosis patients
- Comparison of Total and IgG ABO Isoagglutinin Titers in ABO-Incompatible Organ Transplant Patients: Analysis of Data from a University Hospital Over the Last 5 Years
- Changes of Tetanus Specific IgG, IgM and IgG Subclasses after DPT Vaccination