Obstet Gynecol Sci.
2018 Jan;61(1):71-78. 10.5468/ogs.2018.61.1.71.
Effects of estradiol on HIF-1α expression and trophoblast differentiation in first trimester villous explant cultures
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea. mjohmd@korea.ac.kr
- 2J & L Women's Clinics, Seoul, Korea.
- KMID: 2420154
- DOI: http://doi.org/10.5468/ogs.2018.61.1.71
Abstract
OBJECTIVE
The purpose of this study was to investigate the effects of estradiol on the expression of hypoxia-inducible factor (HIF)-1α and the differentiation of trophoblasts in human first trimester villous explant cultures.
METHODS
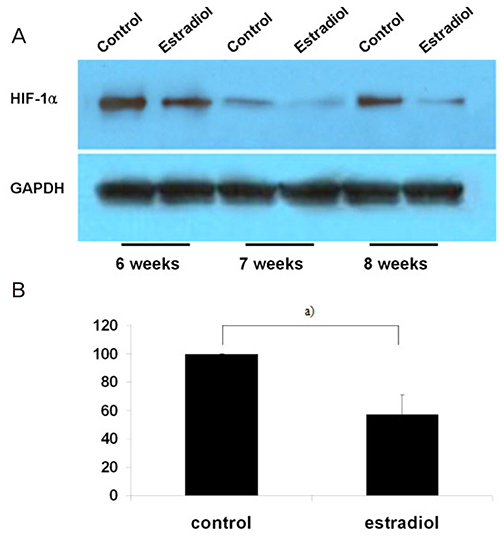
Villous explant cultures were established from first trimester human placentas (6-8 weeks of gestation, n=3). Normal villous tissues were explanted on Matrigel and incubated under 3% O2 tension for 5 days. To evaluate the effects of estradiol on the villous explant cultures, 1 ng/mL of estradiol was added to the culture medium. The morphological integrities and viabilities of the villous explants were monitored. Immunohistochemistry for α5 and α1 integrin was performed to assess differentiation of extravillous trophoblasts (EVTs). Expression of HIF-1α in villous explant cultures was evaluated by western blotting and densitometry.
RESULTS
EVTs emerging from first trimester villous explant cultures formed outgrowths of cells from the distal ends and invaded the surrounding Matrigel. Exposure of villous explants to estradiol resulted in the decreased outgrowth of cells from the distal end and decreased expression of α5 integrin. However, estradiol treatment increased the invasion of villous explants into the surrounding Matrigel, concomitant with the increased expression of α1 integrin, indicating differentiation of EVTs into more invasive EVTs. On western blots, the expression of HIF-1α decreased significantly after treatment with estradiol under 3% O2 tension.
CONCLUSION
Our findings suggest that estradiol may downregulate expression of HIF-1α in placenta, which in turn promote trophoblast differentiation into invasive phenotype.
MeSH Terms
Figure
Reference
-
1. Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993; 91:950–960.
Article2. Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996; 97:540–550.3. Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994; 120:3657–3666.
Article4. Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992; 80:283–285.5. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997; 277:1669–1672.
Article6. Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000; 21:Suppl A. S25–S30.
Article7. Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest. 2000; 105:577–587.8. Zhou Y, Genbacev O, Damsky CH, Fisher SJ. Oxygen regulates human cytotrophoblast differentiation and invasion: implications for endovascular invasion in normal pregnancy and in pre-eclampsia. J Reprod Immunol. 1998; 39:197–213.
Article9. Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004; 19:176–182.
Article10. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004; 5:343–354.
Article11. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003; 9:677–684.
Article12. Maxwell P, Salnikow K. HIF-1: an oxygen and metal responsive transcription factor. Cancer Biol Ther. 2004; 3:29–35.
Article13. Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod. 2000; 63:559–569.14. Matsuura S, Itakura A, Ohno Y, Nakashima Y, Murata Y, Takeuchi M, et al. Effects of estradiol administration on feto-placental growth in rat. Early Hum Dev. 2004; 77:47–56.
Article15. Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995; 16:608–648.
Article16. Cronier L, Guibourdenche J, Niger C, Malassiné A. Oestradiol stimulates morphological and functional differentiation of human villous cytotrophoblast. Placenta. 1999; 20:669–676.
Article17. Nickel EA, Hsieh CH, Chen JG, Schwacha MG, Chaudry IH. Estrogen suppresses cardiac IL-6 after trauma-hemorrhage via a hypoxia-inducible factor 1 alpha-mediated pathway. Shock. 2009; 31:354–358.18. Mukundan H, Kanagy NL, Resta TC. 17-beta estradiol attenuates hypoxic induction of HIF-1alpha and erythropoietin in Hep3B cells. J Cardiovasc Pharmacol. 2004; 44:93–100.19. Dawood MY, Ratnam SS. Serum unconjugated estradiol-17 beta in normal pregnancy measured by radioimmunoassay. Obstet Gynecol. 1974; 44:194–199.20. Rajakumar A, Whitelock KA, Weissfeld LA, Daftary AR, Markovic N, Conrad KP. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia. Biol Reprod. 2001; 64:499–506.21. Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003; 24:199–208.22. Abdul-Karim RW, Nesbitt RE Jr, Drucker MH, Rizk PT. The regulatory effect of estrogens on fetal growth. I. Placental and fetal body weights. Am J Obstet Gynecol. 1971; 109:656–661.23. Storment JM, Meyer M, Osol G. Estrogen augments the vasodilatory effects of vascular endothelial growth factor in the uterine circulation of the rat. Am J Obstet Gynecol. 2000; 183:449–453.
Article24. Ranta T, Stenman UH, Unnérus HA, Rossi J, Seppälä M. Maternal plasma prolactin levels in preeclampsia. Obstet Gynecol. 1980; 55:428–430.25. Allen EI, Lachelin GC. A comparison of plasma levels of progesterone, oestradiol, unconjugated oestriol and total oestriol with urinary total oestrogen levels in clinical obstetric practice. Br J Obstet Gynaecol. 1978; 85:278–292.
Article26. Walsh SW. Progesterone and estradiol production by normal and preeclamptic placentas. Obstet Gynecol. 1988; 71:222–226.
Article27. Rosing U, Carlström K. Serum levels of unconjugated and total oestrogens and dehydroepiandrosterone, progesterone and urinary oestriol excretion in pre-eclampsia. Gynecol Obstet Invest. 1984; 18:199–205.
Article28. Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol. 1999; 180:60–63.
Article29. Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, et al. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol. 2003; 32:455–460.
Article30. Smith GV, Smith OW. Estrogen and progestin metabolism in pregnancy. III. The effect of hormone administration in pre-eclampsia. J Clin Endocrinol. 1941; 1:477–484.31. Mizutani S, Kurauchi O, Ito Y, Narita O, Tomoda Y. Positive effect of estradiol and progesterone in severe pre-eclampsia. Exp Clin Endocrinol. 1988; 92:161–170.
Article32. Vatten LJ, Romundstad PR, Trichopoulos D, Skjaerven R. Pre-eclampsia in pregnancy and subsequent risk for breast cancer. Br J Cancer. 2002; 87:971–973.
Article33. Ekbom A, Trichopoulos D, Adami HO, Hsieh CC, Lan SJ. Evidence of prenatal influences on breast cancer risk. Lancet. 1992; 340:1015–1018.
Article34. Takanashi K, Honma T, Kashiwagi T, Honjo H, Yoshizawa I. Detection and measurement of urinary 2-hydroxyestradiol 17-sulfate, a potential placental antioxidant during pregnancy. Clin Chem. 2000; 46:373–378.
Article35. Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006; 291:R1085–R1093.36. Molvarec A, Vér A, Fekete A, Rosta K, Derzbach L, Derzsy Z, et al. Association between estrogen receptor alpha (ESR1) gene polymorphisms and severe preeclampsia. Hypertens Res. 2007; 30:205–211.
Article37. Maruyama A, Nakayama T, Sato N, Mizutani Y, Furuya K, Yamamoto T. Association study using single nucleotide polymorphisms in the estrogen receptor beta (ESR2) gene for preeclampsia. Hypertens Res. 2004; 27:903–909.38. Pribluda VS, Gubish ER Jr, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000; 19:173–179.39. LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002; 62:3691–3697.40. Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003; 3:363–375.
Article41. Casey ML, MacDonald PC. Characterization of catechol-O-methyltransferase activity in human uterine decidua vera tissue. Am J Obstet Gynecol. 1983; 145:453–457.
Article42. Berg D, Sonsalla R, Kuss E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh). 1983; 103:282–288.
Article43. Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Catechol-o-methyl transferase activity in the human term placenta. Am J Perinatol. 1988; 5:121–127.
Article44. Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008; 453:1117–1121.
Article45. Yun SP, Lee MY, Ryu JM, Song CH, Han HJ. Role of HIF-1alpha and VEGF in human mesenchymal stem cell proliferation by 17beta-estradiol: involvement of PKC, PI3K/Akt, and MAPKs. Am J Physiol Cell Physiol. 2009; 296:C317–C326.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Epidermal Growth Factor, Transforming Growth Factor-alphaand Epidermal Growth Factor Receptor in Human Trophoblast and Decidua
- Hypoxia-Inducible Factor 1α Regulates the Transforming Growth Factor β1/SMAD Family Member 3 Pathway to Promote Breast Cancer Progression
- Hypoxia Inducible Factor-1α Directly Induces the Expression of Receptor Activator of Nuclear Factor-κB Ligand in Chondrocytes
- Effects of Histone Deacetylase Inhibitor (Valproic Acid) on the Expression of Hypoxia-inducible Factor-1 Alpha in Human Retinal Müller Cells
- Increased Expression of Thymosin β₄ Is Independently Correlated with Hypoxia Inducible Factor-1α (HIF-1α) and Worse Clinical Outcome in Human Colorectal Cancer