Yeungnam Univ J Med.
2018 Jun;35(1):1-6. 10.12701/yujm.2018.35.1.1.
Beta-amyloid imaging in dementia
- Affiliations
-
- 1Department of Nuclear Medicine, Yeungnam University College of Medicine, Daegu, Korea. cka52@yumail.ac.kr
- KMID: 2415727
- DOI: http://doi.org/10.12701/yujm.2018.35.1.1
Abstract
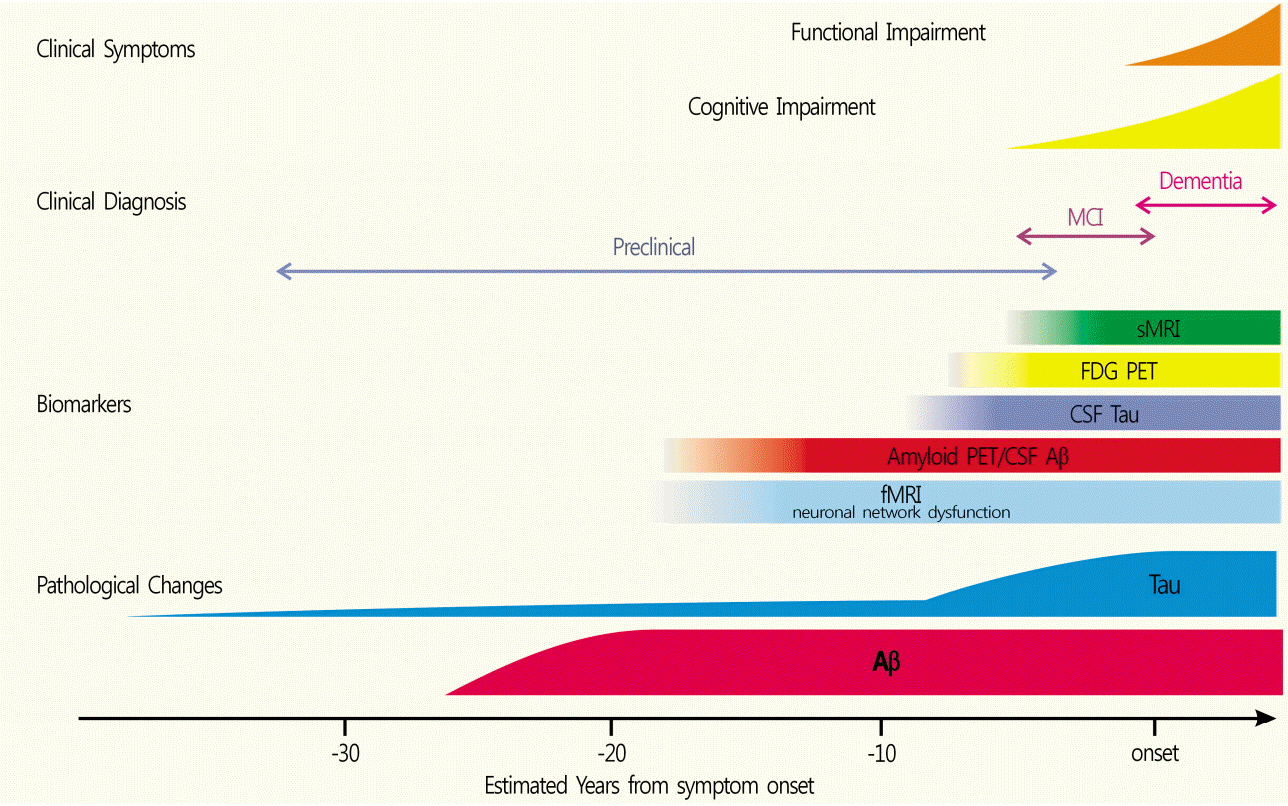
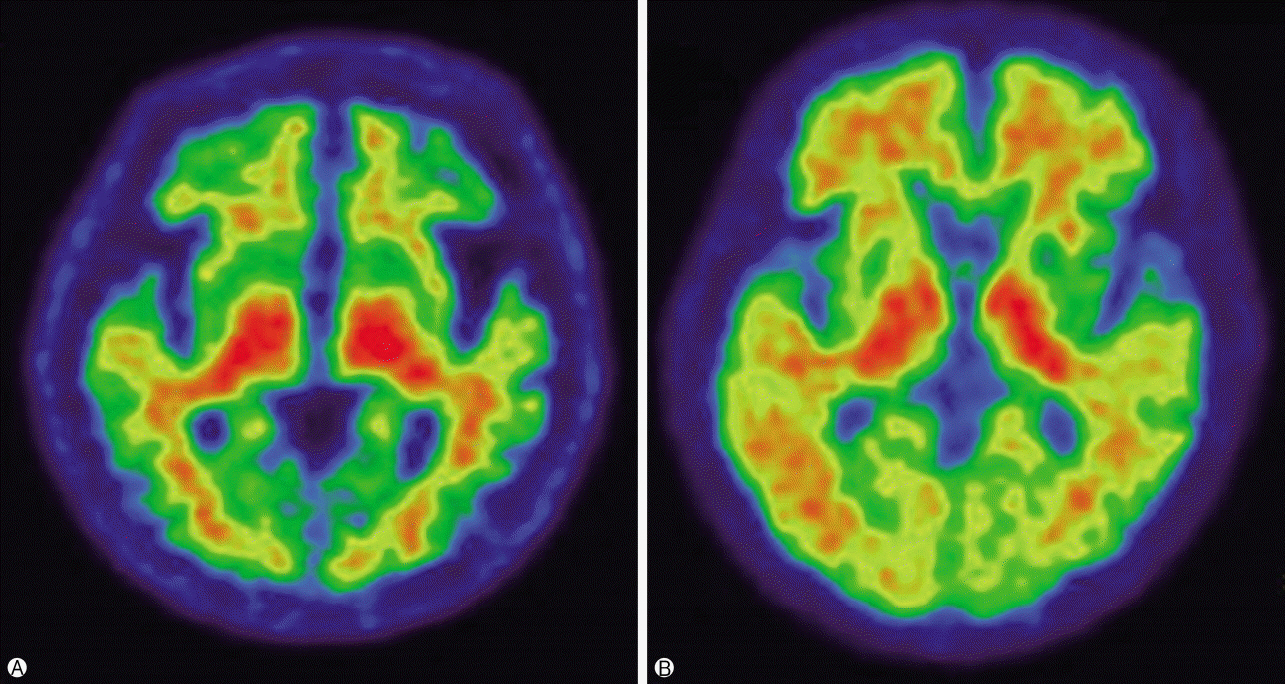
- Alzheimer's disease (AD) is a neurodegenerative disorder associated with extracellular plaques, composed of amyloid-beta (Aβ), in the brain. Although the precise mechanism underlying the neurotoxicity of Aβ has not been established, Aβ accumulation is the primary event in a cascade of events that lead to neurofibrillary degeneration and dementia. In particular, the Aβ burden, as assessed by neuroimaging, has proved to be an excellent predictive biomarker. Positron emission tomography, using ligands such as ¹¹C-labeled Pittsburgh Compound B or ¹â¸F-labeled tracers, such as ¹â¸F-florbetaben, ¹â¸F-florbetapir, and ¹â¸F-flutemetamol, which bind to Aβ deposits in the brain, has been a valuable technique for visualizing and quantifying the deposition of Aβ throughout the brain in living subjects. Aβ imaging has very high sensitivity for detecting AD pathology. In addition, it can predict the progression from mild cognitive impairment to AD, and contribute to the development of disease-specific therapies.
MeSH Terms
Figure
Reference
-
1. Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010; 362:329–44.
Article2. Yoshiyama Y, Lee VM, Trojanowski JQ. Therapeutic strategies for tau mediated neurodegeneration. J Neurol Neurosurg Psychiatry. 2013; 84:784–95.
Article3. Won WY, Lee CU. Biomarkers for Alzheimer’s dementia: focus on neuroimaging. Korean J Biol Psychiatry. 2011; 18:72–79. Korean.4. Villemagne VL, Doré V, Bourgeat P, Burnham SC, Laws S, Salvado O, et al. Aβ-amyloid and tau imaging in dementia. Semin Nucl Med. 2017; 47:75–88.5. Murphy MP, LeVine H 3rd. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010; 19:311–23.6. Harper JD, Lansbury PT Jr. Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997; 66:385–407.
Article7. Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992; 359:325–7.
Article8. Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience. 2001; 105:99–107.
Article9. Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003; 62:1087–95.
Article10. Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol. 2006; 575:5–10.11. Villemagne VL, Cappai R, Barnham KJ, Cherny RA, Opazo C, Novakovic KE, et al. The Aβ centric pathway of Alzheimer’s disease. In : Barrow CJ, Small DH, editors. Abeta peptide and Alzheimer’s disease: celebrating a century of research. London: Springer;2007. p. 5–32.12. Hardy J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997; 20:154–9.
Article13. Kim JS, Oh SJ, Moon DH. Molecular imaging in neurodegenerative diseases. J Korean Med Assoc. 2009; 52:151–67. Korean.
Article14. Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging betaamyloid pathology. JAMA. 2011; 305:275–83.
Article15. Wolk DA, Grachev ID, Buckley C, Kazi H, Grady MS, Trojanowski JQ, et al. Association between in vivo fluorine 18-labeled flutemetamol amyloid positron emission tomography imaging and in vivo cerebral cortical histopathology. Arch Neurol. 2011; 68:1398–403.
Article16. Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004; 55:306–19.
Article17. Kemppainen NM, Aalto S, Wilson IA, Någren K, Helin S, Brück A, et al. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006; 67:1575–80.
Article18. Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011; 77:2034–42.
Article19. Lehmann M, Ghosh PM, Madison C, Laforce R Jr, Corbetta-Rastelli C, Weiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013; 136:844–58.
Article20. Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, Maye J, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012; 27:965–73.
Article21. Graff-Radford J, Boeve BF, Pedraza O, Ferman TJ, Przybelski S, Lesnick TG, et al. Imaging and acetylcholinesterase inhibitor response in dementia with Lewy bodies. Brain. 2012; 135:2470–7.
Article22. Gomperts SN, Locascio JJ, Rentz D, Santarlasci A, Marquie M, Johnson KA, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013; 80:85–91.
Article23. Xia C, Dickerson BC. Multimodal PET imaging of amyloid and tau pathology in Alzheimer disease and non-Alzheimer disease dementias. PET Clin. 2017; 12:351–9.
Article24. Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010; 31:1275–83.
Article25. Wang F, Gordon BA, Ryman DC, Ma S, Xiong C, Hassenstab J, et al. Cerebral amyloidosis associated with cognitive decline in autosomal dominant Alzheimer disease. Neurology. 2015; 85:790–8.
Article26. Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015; 313:1924–38.27. Jack CR Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009; 132:1355–65.
Article28. Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013; 9:e106. –9.
Article29. Bosscher L, Scheltens P. MRI of the medial temporal lobe for the diagnosis of Alzheimer’s disease. In : Qizilbash N, editor. Evidence-based dementia practice. Osney Mead, Oxford;Malden, MA: Blackwell Science;2002. p. 154–62.30. Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008; 49:390–8.
Article31. Arbizu J, García-Ribas G, Carrió I, Garrastachu P, Martínez-Lage P, Molinuevo JL. Recommendations for the use of PET imaging biomarkers in the diagnosis of neurodegenerative conditions associated with dementia: SEMNIM and SEN consensus. Rev Esp Med Nucl Imagen Mol. 2015; 34:303–13.32. Sánchez-Juan P, Ghosh PM, Hagen J, Gesierich B, Henry M, Grinberg LT, et al. Practical utility of amyloid and FDG-PET in an academic dementia center. Neurology. 2014; 82:230–8.
Article33. Barthel H, Sabri O. Clinical use and utility of amyloid imaging. J Nucl Med. 2017; 58:1711–7.
Article34. Masters CL, Beyreuther K. Alzheimer’s centennial legacy: prospects for rational therapeutic intervention targeting the Abeta amyloid pathway. Brain. 2006; 129:2823–39.
Article35. Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-tomoderate Alzheimer’s disease. N Engl J Med. 2014; 370:322–33.
Article36. Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016; 537:50–6.
Article37. Sperling RA, Jack CR Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011; 3:111–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship Between Sleep and Alzheimer’s Dementia
- beta-Amyloid Imaging Probes
- The Effects of Psychotropic Drugs on the beta-Amyloid-Induced Cytotoxicity in PC12 Cells
- Prospect of Geriatric Psychiatric Research: Research on Alzheimer's Disease
- Anti-Amyloid Imaging Abnormality in the Era of Anti-Amyloid Beta Monoclonal Antibodies: Recent Updates for the Radiologist