J Vet Sci.
2016 Jun;17(2):235-242. 10.4142/jvs.2016.17.2.235.
Hematoporphyrin monomethyl ether combined with He-Ne laser irradiation-induced apoptosis in canine breast cancer cells through the mitochondrial pathway
- Affiliations
-
- 1Department of Clinic Veterinary Medicine, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China. abliuyun@yeah.net
- 2Department of Veterinary Medicine, College of Animal Science and Veterinary Medicine, Qingdao Agricultural University, Qingdao 266109, China.
- 3College of Animal Science and Technology, Northeast Agricultural University, Harbin 150030, China.
- 4Synergetic Innovation Center of Food Safety and Nutrition, Northeast Agricultural University, Harbin 150030, China.
- 5Department of Medicine, McMaster University, Hamilton, L8S 4L8, Canada.
- KMID: 2413173
- DOI: http://doi.org/10.4142/jvs.2016.17.2.235
Abstract
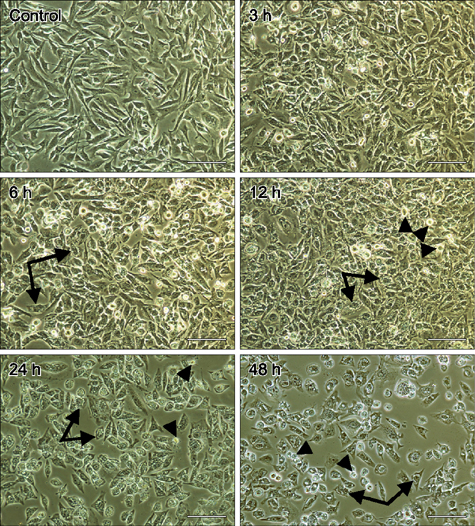
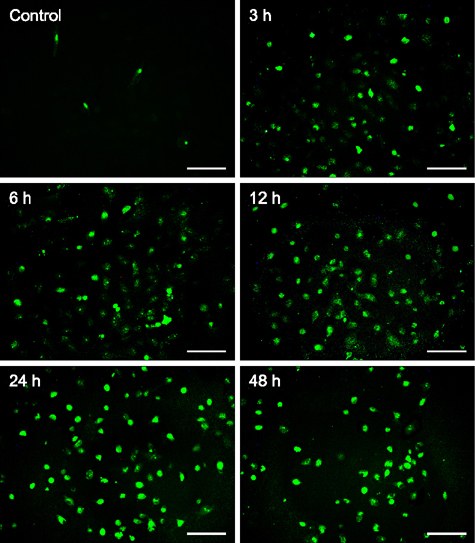
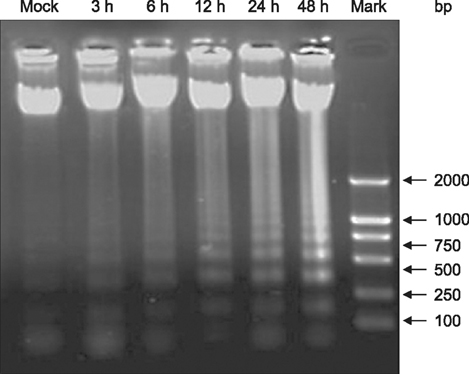
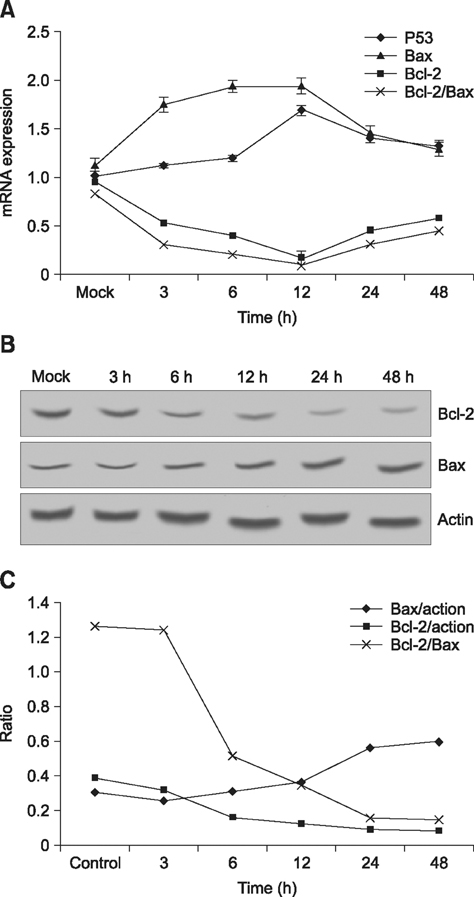
- Hematoporphyrin monomethyl ether (HMME) combined with He-Ne laser irradiation is a novel and promising photodynamic therapy (PDT)-induced apoptosis that can be applied in vitro on canine breast cancer cells. However, the exact pathway responsible for HMME-PDT in canine breast cancer cells remains unknown. CHMm cells morphology and apoptosis were analyzed using optical microscope, terminal deoxynucleotidyl transferase dUTP nick end labeling fluorescein staining and DNA ladder assays. Apoptotic pathway was further confirmed by Real-time-polymerase chain reaction and Western blotting assays. Our results showed that HMME-PDT induced significant changes in cell morphology, such as formation of cytoplasmic vacuoles and the gradual rounding of cells coupled with decreased size and detachment. DNA fragmentation and cell death was shown to occur in a time-dependent manner. Furthermore, HMME-PDT increased the activities of caspase-9 and caspase-3, and released cytochrome c from mitochondria into the cytoplasm. HMME-PDT also significantly increased both mRNA and protein levels of Bax and decreased P53 gene expression in a time-dependent manner, while the mRNA and protein expression of Bcl-2 were repressed. These alterations suggest that HMME-PDT induced CHMm cell apoptosis via the mitochondrial apoptosis pathway and had anti-canine breast cancer effects in vitro.
Keyword
MeSH Terms
-
Animals
*Apoptosis/radiation effects
Breast Neoplasms/etiology/surgery/*veterinary
Caspase 3/metabolism
Caspase 9/metabolism
Cell Line, Tumor
Cytochromes c/metabolism
Dog Diseases/etiology/*surgery
Dogs
Gene Expression/drug effects/radiation effects
Helium
Hematoporphyrins/*pharmacology
*Lasers
Mitochondria/*drug effects/metabolism/*radiation effects
Neon
Photosensitizing Agents/pharmacology
Hematoporphyrins
Photosensitizing Agents
Helium
Neon
Cytochromes c
Caspase 3
Caspase 9
Figure
Reference
-
1. Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003; 39:615–647.
Article2. Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans – man and his best friend share more than companionship. Chromosome Res. 2008; 16:145–154.
Article3. Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007; 1776:86–107.
Article4. Dai W, Li X, Zeng J, Liu F, Gu Y. Influence of combination form of hematoporphyrin monomethyl ether with different kinds of serum protein on its intracellular distribution. Zhongguo ji guang yi xue za zhi. 2006; 3:137–140.5. Ding X, Xu Q, Liu F, Zhou P, Gu Y, Zeng J, An J, Dai W, Li X. Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett. 2004; 216:43–54.
Article6. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998; 90:889–905.
Article7. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article8. Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai). 2005; 37:719–727.
Article9. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998; 281:1309–1312.
Article10. Haga S, Nakayama M, Tatsumi K, Maeda M, Imai S, Umesako S, Yamamoto H, Hilgers J, Sarkar NH. Overexpression of the p53 gene product in canine mammary tumors. Oncol Rep. 2001; 8:1215–1219.
Article11. Hemann MT, Lowe SW. The p53-Bcl-2 connection. Cell Death Differ. 2006; 13:1256–1259.
Article12. Kessel D, Castelli M. Evidence that bcl-2 is the target of three photosensitizers that induce a rapid apoptotic response. Photochem Photobiol. 2001; 74:318–322.
Article13. Kessel D, Reiners JJ Jr. Apoptotic response to photodynamic therapy versus the Bcl-2 antagonist HA14-1. Photochem Photobiol. 2002; 76:314–319.
Article14. Klopfleisch R, Lenze D, Hummel M, Gruber AD. The metastatic cascade is reflected in the transcriptome of metastatic canine mammary carcinomas. Vet J. 2011; 190:236–243.
Article15. Kumaraguruparan R, Prathiba D, Nagini S. Of humans and canines: immunohistochemical analysis of PCNA, Bcl-2, p53, cytokeratin and ER in mammary tumours. Res Vet Sci. 2006; 81:218–224.
Article16. Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001; 276:47379–47386.17. Lee SJ, Kim MS, Park JY, Woo JS, Kim YK. 15-Deoxy-Δ12,14-prostaglandin J2 induces apoptosis via JNK-mediated mitochondrial pathway in osteoblastic cells. Toxicology. 2008; 248:121–129.
Article18. Li HT, Song XY, Yang C, Li Q, Tang D, Tian WR, Liu Y. Effect of hematoporphyrin monomethyl ether-mediated PDT on the mitochondria of canine breast cancer cells. Photodiagnosis Photodyn Ther. 2013; 10:414–421.
Article19. Liu Y, Ma XQ, Jin P, Li HT, Zhang RR, Ren XL, Wang HB, Tang D, Tian WR. Apoptosis induced by hematoporphyrin monomethyl ether combined with He-Ne laser irradiation in vitro on canine breast cancer cells. Vet J. 2011; 188:325–330.
Article20. Ma X, Jing P, Ren X, Zhang R, Liu Y. The research of PDT to exosomatic lethal effect of canine breast tumor cells lines CHMm. Laser J. 2009; 2:81–82.21. Meek DW. The p53 response to DNA damage. DNA Repair (Amst). 2004; 3:1049–1056.
Article22. Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998; 252:1–15.
Article23. Moore KJ, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994; 152:2930–2937.24. Nakagawa T, Watanabe M, Ohashi E, Uyama R, Takauji S, Mochizuki M, Nishimura R, Ogawa H, Sugano S, Sasaki N. Cyclopedic protein expression analysis of cultured canine mammary gland adenocarcinoma cells from six tumours. Res Vet Sci. 2006; 80:317–323.
Article25. Nakamura Y. Isolation of p53-target genes and their functional analysis. Cancer Sci. 2004; 95:7–11.
Article26. Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003; 112:481–490.27. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45.
Article28. Pinho SS, Carvalho S, Cabral J, Reis CA, Gärtner F. Canine tumors: a spontaneous animal model of human carcinogenesis. Transl Res. 2012; 159:165–172.
Article29. Pinthus JH, Bogaards A, Weersink R, Wilson BC, Trachtenberg J. Photodynamic therapy for urological malignancies: past to current approaches. J Urol. 2006; 175:1201–1207.
Article30. Sayan BS, Sayan AE, Knight RA, Melino G, Cohen GM. p53 is cleaved by caspases generating fragments localizing to mitochondria. J Biol Chem. 2006; 281:13566–13573.
Article31. Setoguchi A, Sakai T, Okuda M, Minehata K, Yazawa M, Ishizaka T, Watari T, Nishimura R, Sasaki N, Hasegawa A, Tsujimoto H. Aberrations of the p53 tumor suppressor gene in various tumors in dogs. Am J Vet Res. 2001; 62:433–439.
Article32. Sharman WM, Allen CM, van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999; 4:507–517.
Article33. Song K, Kong B, Li L, Yang Q, Wei Y, Qu X. Intraperitoneal photodynamic therapy for an ovarian cancer ascite model in Fischer 344 rat using hematoporphyrin monomethyl ether. Cancer Sci. 2007; 98:1959–1964.
Article34. Thomas R, Smith KC, Ostrander EA, Galibert F, Breen M. Chromosome aberrations in canine multicentric lymphomas detected with comparative genomic hybridisation and a panel of single locus probes. Br J Cancer. 2003; 89:1530–1537.
Article35. Uva P, Aurisicchio L, Watters J, Loboda A, Kulkarni A, Castle J, Palombo F, Viti V, Mesiti G, Zappulli V, Marconato L, Abramo F, Ciliberto G, Lahm A, La Monica N, de Rinaldis E. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics. 2009; 10:135.
Article36. Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol. 1994; 32:5–8.
Article37. Wang L, Dai W, Li J, Liu F, Gu Y, Li X, Zheng J. Quantitative study of HMME subcellular localization via confocal fluorescence microscopy. Laser J. 2004; 2:77–79.38. Wilson BC. Photodynamic therapy for cancer: principles. Can J Gastroenterol. 2002; 16:393–396.
Article39. Yang WY, Liu CH, Chang CJ, Lee CC, Chang KJ, Lin CT. Proliferative activity, apoptosis and expression of oestrogen receptor and Bcl-2 oncoprotein in canine mammary gland tumours. J Comp Pathol. 2006; 134:70–79.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons
- A Novel Chenodeoxycholic Derivative HS-1200 Enhances Radiation-induced Apoptosis in Human MCF-7 Breast Cancer Cells
- Role of Annexin A5 on Mitochondria-Dependent Apoptosis Induced by Tetramethoxystilbene in Human Breast Cancer Cells
- The Role of Nitric Oxide (NO) in UVB-induced Apoptosis in HaCaT Keratinocytes
- Alkyl Cinnamates Induce Protein Kinase C Translocation and Anticancer Activity against Breast Cancer Cells through Induction of the Mitochondrial Pathway of Apoptosis