J Vet Sci.
2016 Sep;17(3):361-368. 10.4142/jvs.2016.17.3.361.
Seroprevalence and associated risk factors of pseudorabies in Shandong province of China
- Affiliations
-
- 1College of Animal Science and Technology, Shandong Agricultural University, Tai'an 271018, China. liusid@sdau.edu.cn
- KMID: 2413136
- DOI: http://doi.org/10.4142/jvs.2016.17.3.361
Abstract
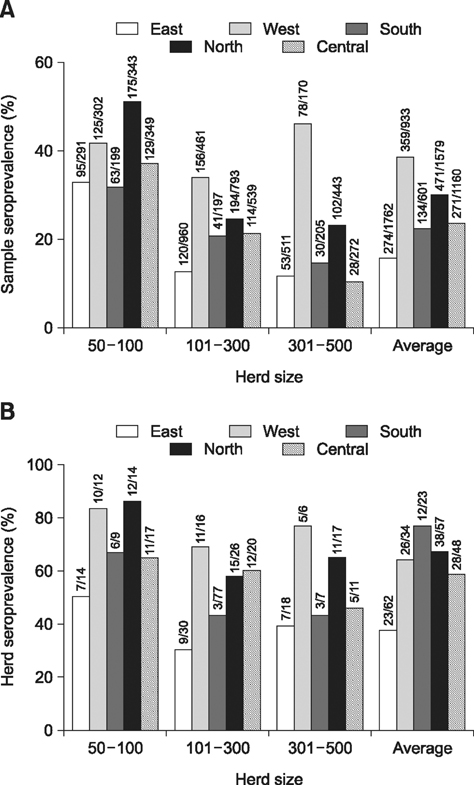
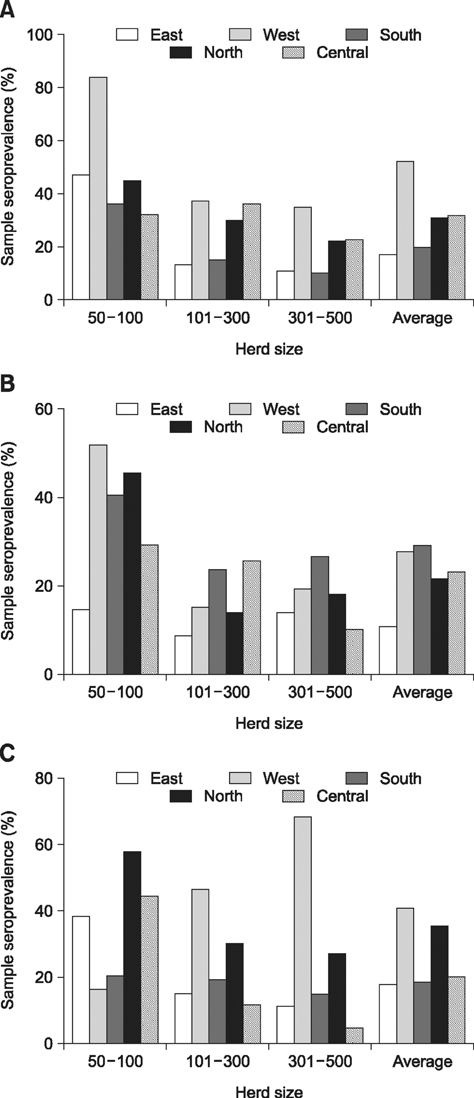
- A cross-sectional serological study was conducted in Shandong province of China to determine the seroprevalence and risk factors associated with seropositivity due to pseudorabies virus (PRV) infection in small- and medium-sized farrow-to-finish herds following outbreaks of variant PRV strains. A total of 6,035 blood samples from 224 randomly selected herds were screened. The results showed that 25.0% of the herds and 56.7% of the serum samples were seropositive for field strains of PRV. Herds consisting of 50-100 breeding sows had higher herd seroprevalence and serum sample seroprevalence than larger herds. Both the highest herd seroprevalence and highest serum sample seroprevalence were observed in western Shandong, followed northern Shandong. Based on univariate analysis, the following risk factors were utilized in subsequent multivariable logistic regression analysis: region, herd size, weight of purchased gilts, and all-in/all-out practice. Upon multivariate analysis, region, herd size, weight of purchased gilts and all-in/all-out practice were significantly associated with PRV herd seropositivity. These findings indicate that we are facing a serious situation in the prevention and control of pseudorabies. The results could help predict the next outbreak and set out control measures.
MeSH Terms
Figure
Reference
-
1. An TQ, Peng JM, Tian ZJ, Zhao HY, Li N, Liu YM, Chen JZ, Leng CL, Sun Y, Chang D, Tong GZ. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis. 2013; 19:1749–1755.
Article2. Boelaert F, Deluyker H, Maes D, Godfroid J, Raskin A, Varewijck H, Pensaert M, Nauwynck H, Castryck F, Miry C, Robijns JM, Hoet B, Segers E, Van Vlaenderen I, Robert A, Koenen F. Prevalence of herds with young sows seropositive to pseudorabies (Aujeszky's disease) in northern Belgium. Prev Vet Med. 1999; 41:239–255.
Article3. Brittle EE, Reynolds AE, Enquist LW. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol. 2004; 78:12951–12963.
Article4. Deen J, Erickson GA, Scherba G. A retrospective study of factors associated with eliminating circulating pseudorabies virus in sow herds. Swine Health Prod. 1999; 7:147–150.5. Doyle LP, Gordon AW, Abernethy DA, Stevens K. Bovine tuberculosis in Northern Ireland: risk factors associated with time from post-outbreak test to subsequent herd breakdown. Prev Vet Med. 2014; 116:47–55.
Article6. Ge X, Wang F, Guo X, Yang H. Porcine circovirus type 2 and its associated diseases in China. Virus Res. 2012; 164:100–106.
Article7. Gu Z, Dong J, Wang J, Hou C, Sun H, Yang W, Bai J, Jiang P. A novel inactivated gE/gI deleted pseudorabies virus (PRV) vaccine completely protects pigs from an emerged variant PRV challenge. Virus Res. 2015; 195:57–63.
Article8. Gut M, Jacobs L, Tyborowska J, Szewczyk B, Bienkowska-Szewczyk K. A highly specific and sensitive competitive enzyme-linked immunosorbent assay (ELISA) based on baculovirus expressed pseudorabies virus glycoprotein gE and gI complex. Vet Microbiol. 1999; 69:239–249.
Article9. Hu D, Zhang Z, Lv L, Xiao Y, Qu Y, Ma H, Niu Y, Wang G, Liu S. Outbreak of variant pseudorabies virus in Bartha-K61-vaccinated piglets in central Shandong Province, China. J Vet Diagn Invest. 2015; 27:600–605.
Article10. Ketusing N, Reeves A, Portacci K, Yano T, Olea-Popelka F, Keefe T, Salman M. Evaluation of strategies for the eradication of pseudorabies virus (Aujeszky's disease) in commercial swine farms in Chiang-Mai and Lampoon Provinces, Thailand, using a simulation disease spread model. Transbound Emerg Dis. 2014; 61:169–176.
Article11. Kinker DR, Swenson SL, Wu LL, Zimmerman JJ. Evaluation of serological tests for the detection of pseudorabies gE antibodies during early infection. Vet Microbiol. 1997; 55:99–106.
Article12. Leontides L, Ewald C, Willeberg P. Herd risk factors for serological evidence of Aujeszky's disease virus infection of breeding sows in northern Germany (1990-1991). Zentralbl Veterinarmed B. 1994; 41:554–560.
Article13. Lipowski A. Evaluation of efficacy and safety of Aujeszky's disease vaccines. Pol J Vet Sci. 2006; 9:75–79.14. Luo Y, Li N, Cong X, Wang CH, Du M, Li L, Zhao B, Yuan J, Liu DD, Li S, Li Y, Sun Y, Qiu HJ. Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet Microbiol. 2014; 174:107–115.
Article15. Medveczky I, Lomniczi L. Experiences of the eradication of Aujeszky's disease in Hungary between 1976-1991. In : Proceedings of the 14th International Pig Veterinary Society Congress; 7-10 July 1996; Bologna, Italy.16. Mettenleiter TC. Immunobiology of pseudorabies (Aujeszky's disease). Vet Immunol Immunopathol. 1996; 54:221–229.
Article17. Monger VR, Stegeman JA, Koop G, Dukpa K, Tenzin T, Loeffen WLA. Seroprevalence and associated risk factors of important pig viral diseases in Bhutan. Prev Vet Med. 2014; 117:222–232.
Article18. Morrison RB, Marsh WE, Anderson PL, Thawley DG. Factors associated with the seroprevalence of pseudorabies virus in breeding swine from quarantined herds. J Am Vet Med Assoc. 1991; 199:580–583.19. Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C. Pseudorabies virus in wild swine: a global perspective. Arch Virol. 2011; 156:1691–1705.
Article20. Rashid MH, Xue C, Islam MT, Islam MR, Cao Y. Risk factors associated with infectious bursal disease in commercial chickens in Bangladesh. Prev Vet Med. 2013; 111:181–185.
Article21. Rodríguez-Buenfil JC, Alvarez-Fleites M, Alzina-Lopez A, Arjona-Torres MG, Segura-Correa JC, Villegas-Pérez S. Risk factors for Aujeszky's disease in pig herds and detection of field virus antibodies in fattening pigs in the state of Yucatan, Mexico. Prev Vet Med. 2002; 53:205–213.
Article22. Solymosi N, Reiczigel J, Berke O, Harnos A, Szigeti S, Fodor L, Szigeti G, Boódis K. Spatial risk assessment of herd sero-status of Aujeszky's disease in a county in Hungary. Prev Vet Med. 2004; 65:9–16.
Article23. Stegeman A, Elbers ARW, Loeffen W, De Jong MCM, Tielen MJM. Rate of successful pseudorabies virus introductions in swine breeding herds in the southern Netherlands that participated in an area-wide vaccination programme. Prev Vet Med. 1996; 27:29–41.
Article24. Szpara ML, Tafuri YR, Parsons L, Shamim SR, Verstrepen KJ, Legendre M, Enquist LW. A wide extent of inter-strain diversity in virulent and vaccine strains of alphaherpesviruses. PLoS Pathog. 2011; 7:e1002282.
Article25. Tamba M, Calabrese R, Finelli E, Cordioli P. Risk factors for Aujeszky's-disease seropositivity of swine herds of a region of northern Italy. Prev Vet Med. 2002; 54:203–212.
Article26. Thrusfield MV. Veterinary Epidemiology. London: Butterworths;1986.27. Virtanen S, Nikunen S, Korkeala H. Introduction of infected animals to herds is an important route for the spread of Yersinia enterocolitica infection between pig farms. J Food Prot. 2014; 77:116–121.
Article28. Weigel RM, Austin CC, Siegel AM, Biehl LG, Taft AC. Risk factors associated with the seroprevalence of pseudorabies virus in Illinois swine herds. Prev Vet Med. 1992; 12:1–13.
Article29. Wu R, Bai C, Sun J, Chang S, Zhang X. Emergence of virulent pseudorabies virus infection in northern China. J Vet Sci. 2013; 14:363–365.
Article30. Yu X, Zhou Z, Hu D, Zhang Q, Han T, Li X, Gu X, Yuan L, Zhang S, Wang B, Qu P, Liu J, Zhai X, Tian K. Pathogenic pseudorabies virus, China, 2012. Emerg Infect Dis. 2014; 20:102–104.
Article31. Zhou S, Guo F, Li L, Zhou Y, Lei Y, Hu Y, Su H, Chen X, Yin P, Jian X. Multiple logistic regression analysis of risk factors for carcinogenesis of oral submucous fibrosis in mainland China. Int J Oral Maxillofac Surg. 2008; 37:1094–1098.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Seroprevalence of Toxoplasma gondii Among Primary School Children in Shandong Province, China
- Epidemiological investigation of porcine pseudorabies virus and its coinfection rate in Shandong Province in China from 2015 to 2018
- Correlation Between Bone Marrow Blasts Counts With Flow Cytometry and Morphological Analysis in Myelodysplastic Syndromes
- Atypical Meningoencephalomyelitis Following Varicella Zoster Virus Infection
- Successful Treatment of Hailey-Hailey Disease with Aminolevulinic Acid Photodynamic Therapy