J Pathol Transl Med.
2018 May;52(3):183-190. 10.4132/jptm.2017.10.16.
Erdheim-Chester Disease Involving Lymph Nodes and Liver Clinically Mimicking Lymphoma: A Case Report
- Affiliations
-
- 1Department of Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. leekyoyo@catholic.ac.kr
- 2Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Cancer Research Institute, The Catholic University of Korea, Seoul, Korea.
- KMID: 2412931
- DOI: http://doi.org/10.4132/jptm.2017.10.16
Abstract
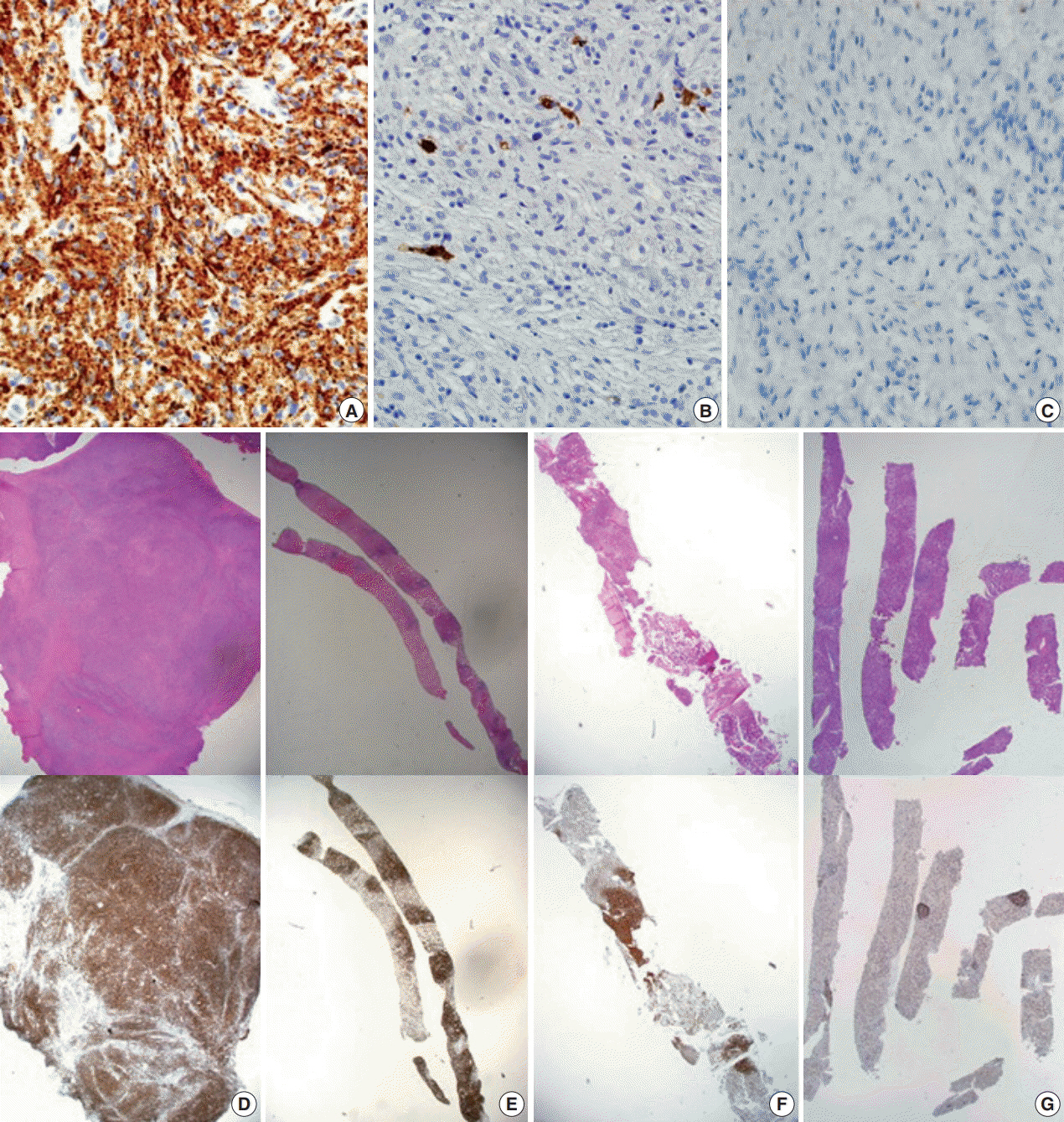
- Erdheim-Chester disease (ECD) is a rare non-Langerhans cell histiocytosis and multisystem disease. First described in 1930, there are no more than 750 cases reported. The etiology remains unknown, but a majority of cases of ECD and Langerhans cell histiocytosis were found to have clonal mutations involving genes of the mitogen-activated protein kinase pathway. We recently encountered a 53-year-old male patient with extensive ECD involving the systemic lymph nodes, pleura, liver, and long bones clinically mimicking malignant lymphoma. Biopsies were performed at multiple sites, including a pleural mass, an external iliac lymph node, bone marrow, and the liver. Based on histopathological and immunohistochemical findings of positivity for CD68 and negativity for CD1a and S-100, the patient was diagnosed with ECD. Interferon-α was administered as the first-line treatment, but the patient rapidly progressed to hepatic failure after 2 months of treatment. We report this rare case of ECD clinically mimicking malignant lymphoma and diagnosed by careful pathological review.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Primary Necrobiotic Xanthogranulomatous Sialadenitis with Submandibular Gland Localization without Skin Involvement
Myunghee Kang, Na Rae Kim, Dong Hae Chung, Jae Yeon Seok, Dong Young Kim
J Pathol Transl Med. 2019;53(4):261-265. doi: 10.4132/jptm.2019.01.08.
Reference
-
1. Campochiaro C, Tomelleri A, Cavalli G, Berti A, Dagna L. Erdheim-Chester disease. Eur J Intern Med. 2015; 26:223–9.
Article2. Lim J, Kim KH, Suh KJ, et al. A unique case of Erdheim-Chester disease with axial skeleton, lymph node, and bone marrow involvement. Cancer Res Treat. 2016; 48:415–21.
Article3. Bindra J, Lam A, Lamba R, VanNess M, Boutin RD. Erdheim-Chester disease: an unusual presentation of an uncommon disease. Skeletal Radiol. 2014; 43:835–40.
Article4. Pavlidakey PG, Mohanty A, Kohler LJ, Meyerson HJ. Erdheim-chester disease associated with marginal zone lymphoma and monoclonal proteinemia. Case Rep Hematol. 2011; 2011:941637.
Article5. Haroche J, Amoura Z, Wechsler B, Veyssier-Belot C, Charlotte F, Piette JC. Erdheim-Chester disease. Presse Med. 2007; 36:1663–8.6. Sheu SY, Wenzel RR, Kersting C, Merten R, Otterbach F, Schmid KW. Erdheim-Chester disease: case report with multisystemic manifestations including testes, thyroid, and lymph nodes, and a review of literature. J Clin Pathol. 2004; 57:1225–8.
Article7. Ivan D, Neto A, Lemos L, Gupta A. Erdheim-Chester disease: a unique presentation with liver involvement and vertebral osteolytic lesions. Arch Pathol Lab Med. 2003; 127:e337–9.
Article8. Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014; 124:483–92.
Article9. Cha YJ, Yang WI, Park SH, Koo JS. Rosai-Dorfman disease in the breast with increased IgG4 expressing plasma cells: a case report. Korean J Pathol. 2012; 46:489–93.
Article10. Haroche J, Amoura Z, Charlotte F, et al. Imatinib mesylate for platelet-derived growth factor receptor-beta-positive Erdheim-Chester histiocytosis. Blood. 2008; 111:5413–5.
Article11. Arnaud L, Hervier B, Néel A, et al. CNS involvement and treatment with interferon-alpha are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011; 117:2778–82.12. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016; 127:2672–81.
Article13. Hervier B, Haroche J, Arnaud L, et al. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014; 124:1119–26.14. Emile JF, Diamond EL, Hélias-Rodzewicz Z, et al. Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood. 2014; 124:3016–9.15. Munoz J, Janku F, Cohen PR, Kurzrock R. Erdheim-Chester disease: characteristics and management. Mayo Clin Proc. 2014; 89:985–96.
Article16. Tzoulis C, Schwarzlmuller T, Gjerde IO, et al. Excellent response of intramedullary Erdheim-Chester disease to vemurafenib: a case report. BMC Res Notes. 2015; 8:171.
Article17. Nordmann TM, Juengling FD, Recher M, et al. Trametinib after disease reactivation under dabrafenib in Erdheim-Chester disease with both BRAF and KRAS mutations. Blood. 2017; 129:879–82.18. Cohen-Aubart F, Maksud P, Saadoun D, et al. Variability in the efficacy of the IL1 receptor antagonist anakinra for treating Erdheim-Chester disease. Blood. 2016; 127:1509–12.
Article19. Janku F, Amin HM, Yang D, Garrido-Laguna I, Trent JC, Kurzrock R. Response of histiocytoses to imatinib mesylate: fire to ashes. J Clin Oncol. 2010; 28:e633–6.
Article20. Haroche J, Cohen-Aubart F, Rollins BJ, et al. Histiocytoses: emerging neoplasia behind inflammation. Lancet Oncol. 2017; 18:e113–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erdheim-Chester Disease with Perirenal Masses Containing Macroscopic Fat Tissue
- Erdheim–Chester Disease Involving the Biliary System and Mimicking Immunoglobulin G4-Related Disease: A Case Report
- Commentary on "A Case of Erdheim-Chester Disease with Asymptomatic Renal Involvement"
- A Case of Erdheim-Chester Disease with Bilateral Hydronephrosis
- A Case of Erdheim-Chester Disease Mimicking Systemic Lymphangitic Metastasis