J Vet Sci.
2017 Mar;18(1):39-49. 10.4142/jvs.2017.18.1.39.
Inflammation affects the viability and plasticity of equine mesenchymal stem cells: possible implications in intra-articular treatments
- Affiliations
-
- 1Laboratory of Biochemical Genetics LAGENBIO, Veterinary Hospital, University of Zaragoza, 50013 Zaragoza, Spain. rodellar@unizar.es
- 2Service of Equine Surgery and Medicine, Veterinary Hospital, University of Zaragoza, 50013 Zaragoza, Spain.
- 3Service of Orthopedic Surgery and Traumatology, University Clinical Hospital Lozano Blesa, 50009 Zaragoza, Spain.
- 4Service of Equine Surgery, Veterinary Hospital, Autonomous University of Barcelona, 08193 Barcelona, Spain.
- KMID: 2412586
- DOI: http://doi.org/10.4142/jvs.2017.18.1.39
Abstract
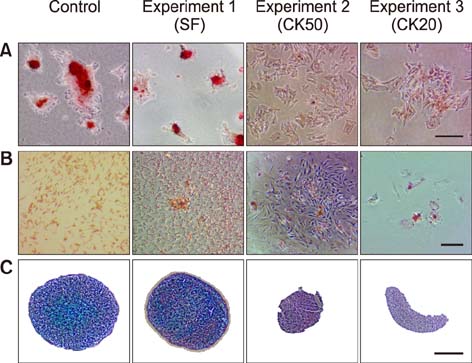
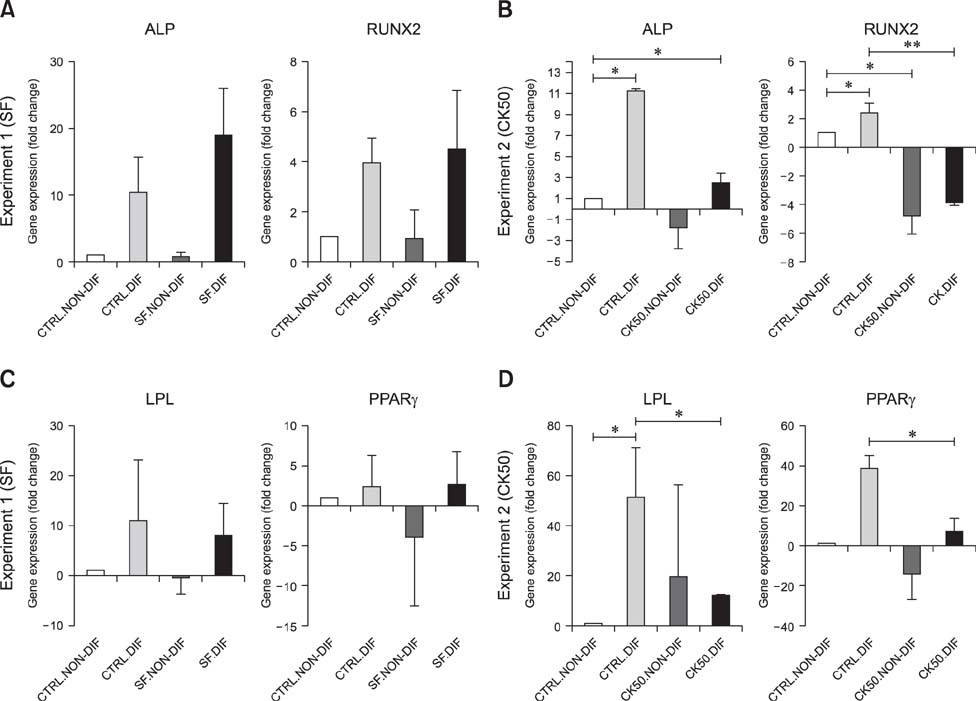
- Mesenchymal stem cells (MSCs) are gaining relevance for treating equine joint injuries because of their ability to limit inflammation and stimulate regeneration. Because inflammation activates MSC immunoregulatory function, proinflammatory priming could improve MSC efficacy. However, inflammatory molecules present in synovial fluid or added to the culture medium might have deleterious effects on MSCs. Therefore, this study was conducted to investigate the effects of inflammatory synovial fluid and proinflammatory cytokines priming on viability and plasticity of equine MSCs. Equine bone marrow derived MSCs (eBM-MSCs) from three animals were cultured for 72 h in media supplemented with: 20% inflammatory synovial fluid (SF); 50 ng/mL IFN-γ and TNF-α (CK50); and 20 ng/mL IFN-γ and TNF-α (CK20). Proliferation assay and expression of proliferation and apoptosis-related genes showed that SF exposed-eBM-MSCs maintained their viability, whereas the viability of CK primed-eBM-MSCs was significantly impaired. Tri-lineage differentiation assay revealed that exposure to inflammatory synovial fluid did not alter eBM-MSCs differentiation potential; however, eBM-MSCs primed with cytokines did not display osteogenic, adipogenic or chondrogenic phenotype. The inflammatory synovial environment is well tolerated by eBM-MSCs, whereas cytokine priming negatively affects the viability and differentiation abilities of eBM-MSCs, which might limit their in vivo efficacy.
Keyword
MeSH Terms
-
Animals
Horse Diseases/*immunology/metabolism
Horses
Inflammation/immunology/metabolism/*veterinary
Injections, Intra-Articular/veterinary
Interferon-gamma/*metabolism
Male
Mesenchymal Stromal Cells/*cytology
Synovial Fluid/*cytology
Tumor Necrosis Factor-alpha/*metabolism
Tumor Necrosis Factor-alpha
Interferon-gamma
Figure
Reference
-
1. Basile RC, Ferraz GC, Carvalho MP, Albernaz RM, Araújo RA, Fagliari JJ, Queiroz-Neto A. Physiological concentrations of acute-phase proteins and immunoglobulins in equine synovial fluid. J Equine Vet Sci. 2013; 33:201–204.
Article2. Casella S, Fazio F, Giannetto C, Giudice E, Piccione G. Influence of transportation on serum concentrations of acute phase proteins in horse. Res Vet Sci. 2012; 93:914–917.
Article3. Cellular, Tissue, and Gene Therapies Advisory Committee. In : Cellular products for joint surface repair. Briefing document of the 38th Meeting of Cellular, Tissue, and Gene Therapies Advisory Committee; 3-4 March 2005; Silver Spring, USA.4. Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013; 36:379–386.
Article5. Cuerquis J, Romieu-Mourez R, François M, Routy JP, Young YK, Zhao J, Eliopoulos N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy. 2014; 16:191–202.
Article6. Dighe AS, Yang S, Madhu V, Balian G, Cui Q. Interferon gamma and T cells inhibit osteogenesis induced by allogeneic mesenchymal stromal cells. J Orthop Res. 2013; 31:227–234.
Article7. Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, Hardouin P, Magne D. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009; 84:499–504.
Article8. Ferris DJ, Frisbie DD, Kisiday JD, McIlwraith CW, Hague BA, Major MD, Schneider RK, Zubrod CJ, Kawcak CE, Goodrich LR. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. 2014; 43:255–265.
Article9. Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2αA) is inhibited by tumor necrosis factor-α. J Biol Chem. 2002; 277:2695–2701.
Article10. Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. Am J Physiol Endocrinol Metab. 2005; 288:E1011–E1018.
Article11. Goodrich LR, Nixon AJ. Medical treatment of osteoarthritis in the horse - a review. Vet J. 2006; 171:51–69.
Article12. Hegewald AA, Ringe J, Bartel J, Krüger I, Notter M, Barnewitz D, Kaps C, Sittinger M. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004; 36:431–438.
Article13. Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009; 361:1570–1583.
Article14. Jacobsen S, Thomsen MH, Nanni S. Concentrations of serum amyloid A in serum and synovial fluid from healthy horses and horses with joint disease. Am J Vet Res. 2006; 67:1738–1742.
Article15. Kolm G, Klein D, Knapp E, Watanabe K, Walter I. Lactoferrin expression in the horse endometrium: relevance in persisting mating-induced endometritis. Vet Immunol Immunopathol. 2006; 114:159–167.
Article16. Krüger JP, Endres M, Neumann K, Stuhlmüller B, Morawietz L, Häupl T, Kaps C. Chondrogenic differentiation of human subchondral progenitor cells is affected by synovial fluid from donors with osteoarthritis or rheumatoid arthritis. J Orthop Surg Res. 2012; 7:10.
Article17. Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009; 17:735–742.
Article18. Leijs MJC, van Buul GM, Lubberts E, Bos PK, Verhaar JAN, Hoogduijn MJ, van Osch GJVM. Effect of arthritic synovial fluids on the expression of immunomodulatory factors by mesenchymal stem cells: an explorative in vitro study. Front Immunol. 2012; 3:231.19. Liu X, Xu Y, Chen S, Tan Z, Xiong K, Li Y, Ye Y, Luo ZP, He F, Gong Y. Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic Biol Med. 2014; 68:234–246.
Article20. Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011; 17:1594–1601.
Article21. Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014; 21:216–225.
Article22. Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-α on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010; 31:1666–1675.
Article23. Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-α. J Biol Chem. 2000; 275:3687–3692.
Article24. Nanes MS, McKoy WM, Marx SJ. Inhibitory effects of tumor necrosis factor-α and interferon-γ on deoxyribonucleic acid and collagen synthesis by rat osteosarcoma cells (ROS 17/2.8). Endocrinology. 1989; 124:339–345.
Article25. Ogueta S, Muñoz J, Obregon E, Delgado-Baeza E, García-Ruiz JP. Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol Cell Endocrinol. 2002; 190:51–63.
Article26. Paterson YZ, Rash N, Garvican ER, Paillot R, Guest DJ. Equine mesenchymal stromal cells and embryo-derived stem cells are immune privileged in vitro. Stem Cell Res Ther. 2014; 5:90.27. Ranera B, Lyahyai J, Romero A, Vázquez FJ, Remacha AR, Bernal ML, Zaragoza P, Rodellar C, Martín-Burriel I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 2011; 144:147–154.
Article28. Ranera B, Ordovás L, Lyahyai J, Bernal ML, Fernandes F, Remacha AR, Romero A, Vázquez FJ, Osta R, Cons C, Varona L, Zaragoza P, Martín-Burriel I, Rodellar C. Comparative study of equine bone marrow and adipose tissue-derived mesenchymal stromal cells. Equine Vet J. 2012; 44:33–42.
Article29. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008; 2:141–150.
Article30. Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010; 184:2321–2328.
Article31. Roberts S, Genever P, McCaskie A, De Bari C. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011; 6:351–366.
Article32. Schlueter AE, Orth MW. Equine osteoarthritis: a brief review of the disease and its causes. Equine Comp Exerc Physiol. 2004; 1:221–231.
Article33. Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005; 23:1357–1366.
Article34. Son TW, Yun SP, Yong MS, Seo BN, Ryu JM, Youn HY, Oh YM, Han HJ. Netrin-1 protects hypoxia-induced mitochondrial apoptosis through HSP27 expression via DCC- and integrin α6β4-dependent Akt, GSK-3β, and HSF-1 in mesenchymal stem cells. Cell Death Dis. 2013; 4:e563.
Article35. van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JAN, Bernsen MR, van Osch GJVM. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012; 20:1186–1196.
Article36. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998; 38:97–120.
Article37. Vézina Audette R, Lavoie-Lamoureux A, Lavoie JP, Laverty S. Inflammatory stimuli differentially modulate the transcription of paracrine signaling molecules of equine bone marrow multipotent mesenchymal stromal cells. Osteoarthritis Cartilage. 2013; 21:1116–1124.
Article38. Wang L, Zhao Y, Liu Y, Akiyama K, Chen C, Qu C, Jin Y, Shi S. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells. 2013; 31:1383–1395.
Article39. Zimmermann JA, McDevitt TC. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy. 2014; 16:331–345.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Articular Cartilage Injury Using Mesenchymal Stem Cells
- Toward a Reconceptualization of Stem Cells from Cellular Plasticity
- Intra-Articular Injection of miR-29a-3p of BMSCs Promotes Cartilage Self-Repairing and Alleviates Pain in the Rat Osteoarthritis
- Cell Biological Characteristics of Adult Stem Cells
- Characterization and clinical application of mesenchymal stem cells from equine umbilical cord blood