J Vet Sci.
2013 Sep;14(3):367-371. 10.4142/jvs.2013.14.3.367.
Characterization and clinical application of mesenchymal stem cells from equine umbilical cord blood
- Affiliations
-
- 1Department of Veterinary Internal Medicine, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. jschae@snu.ac.kr
- 2Adult Stem Cell Research Center, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. kangpub@snu.ac.kr
- 3Laboratory of Stem Cell and Tumor Biology, Department of Veterinary Public Health, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- KMID: 1705569
- DOI: http://doi.org/10.4142/jvs.2013.14.3.367
Abstract
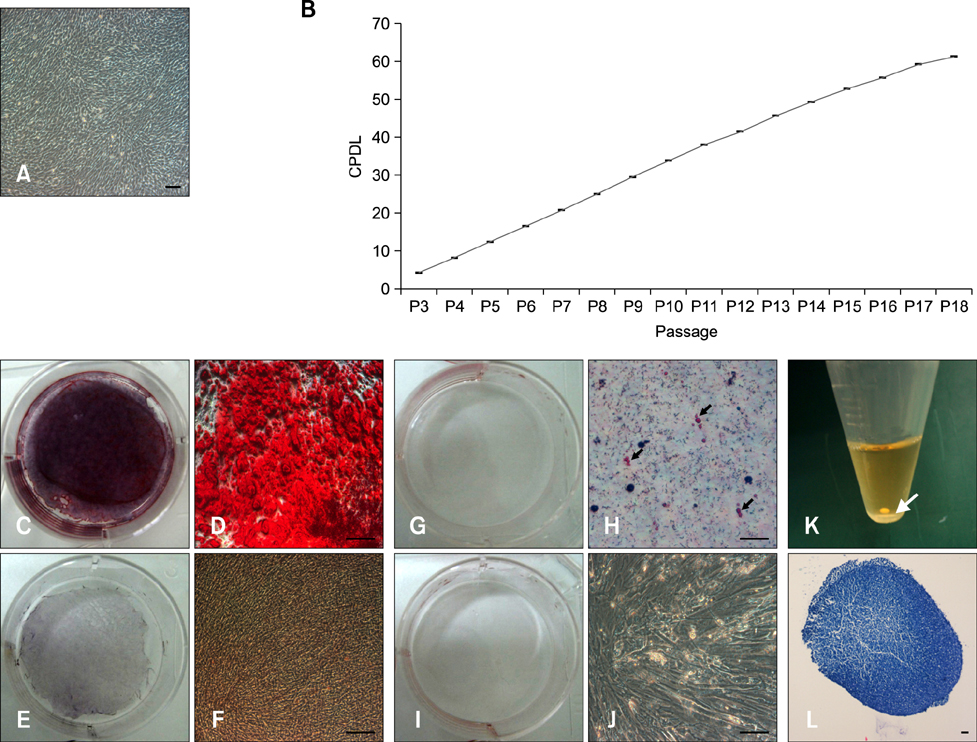
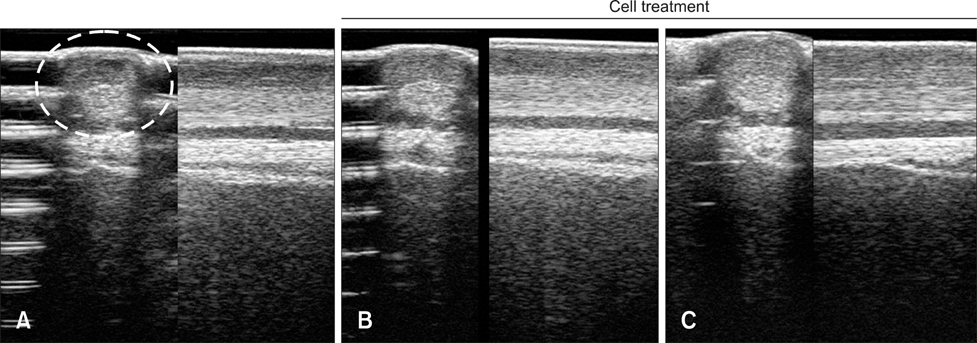
- Tendinitis of the superficial digital flexor tendon (SDFT) is a significant cause of lameness in horses; however, recent studies have shown that stem cells could be useful in veterinary regenerative medicine. Therefore, we isolated and characterized equine umbilical cord blood mesenchymal stem cells (eUCB-MSCs) from equine umbilical cord blood obtained from thoroughbred mares during the foaling period. Horses that had tendinitis of the SDFT were treated with eUCB-MSCs to confirm the therapeutic effect. After eUCB-MSCs transplantation, the core lesion in the SDFT was found to decrease. These results suggest that transplantation using eUCB-MSCs could be another source of cell treatment.
MeSH Terms
Figure
Reference
-
1. Arnhold SJ, Goletz I, Klein H, Stumpf G, Beluche LA, Rohde C, Addicks K, Litzke LF. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am J Vet Res. 2007; 68:1095–1105.
Article2. Crevier-Denoix N, Collobert C, Pourcelot P, Denoix JM, Sanaa M, Geiger D, Bernard N, Ribot X, Bortolussi C, Bousseau B. Mechanical properties of pathological equine superficial digital flexor tendons. Equine Vet J Suppl. 1997; 23:23–26.
Article3. Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005; 23:84–92.
Article4. Domimici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.
Article5. Dyson SJ. Medical management of superficial digital flexor tendonitis: a comparative study in 219 horses (1992-2000). Equine Vet J. 2004; 36:415–419.
Article6. Gargett CE. Identification and characterisation of human endometrial stem/progenitor cells. Aust N Z J Obstet Gynaecol. 2006; 46:250–253.
Article7. Genovese R, Longo K, Berthold B, Jorgensen J. Quantitative sonographic assessment in the clinical management of superficial digital flexor injures in thoroughbred racehorses. AAEP Proceedings. 1997; 43:285–290.8. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RKW. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012; 44:25–32.
Article9. Hoynowski SM, Fry MM, Gardner BM, Leming MT, Tucker JR, Black L, Sand T, Mitchell KE. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem Biophys Res Commun. 2007; 362:347–353.
Article10. Kasashima Y, Takahashi T, Smith RKW, Goodship AE, Kuwano A, Ueno T, Hirano S. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese thoroughbred flat racehorses in 1999. Equine Vet J. 2004; 36:346–350.
Article11. Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008; 26:322–331.
Article12. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007; 7:26.
Article13. Koerner J, Nesic D, Romero JD, Brehm W, Mainil-Varlet P, Grogan SP. Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells. 2006; 24:1613–1619.
Article14. de Mattos Carvalho A, Alves ALG, de Oliveira PGG, Álvarez LEC, Amorim RL, Hussni CA, Deffune E. Use of adipose tissue-derived mesenchymal stem cells for experimental tendinitis therapy in equines. J Equine Vet Sci. 2011; 31:26–34.
Article15. Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008; 69:928–937.
Article16. Park SB, Seo MS, Kang JG, Chae JS, Kang KS. Isolation and characterization of equine amniotic fluid-derived multipotent stem cells. Cytotherapy. 2011; 13:341–349.
Article17. Smith RKW, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003; 35:99–102.
Article18. Vidal MA, Kilroy GE, Johnson JR, Lopez MJ, Moore RM, Gimble JM. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg. 2006; 35:601–610.
Article19. Williams IF, Heaton A, McCullagh KG. Cell morphology and collagen types in equine tendon scar. Res Vet Sci. 1980; 28:302–310.
Article20. Woo SLY, Hildebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JHC. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999; 367:Suppl. S312–S323.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- ERRATUM: Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors
- Use of Cord Blood Stem Cells in Cell Therapy