J Korean Med Assoc.
2005 Oct;48(10):993-1004. 10.5124/jkma.2005.48.10.993.
Cell Biological Characteristics of Adult Stem Cells
- Affiliations
-
- 1Catholic High-Performance Cell Therapy Center, The Catholic University of Korea, School of Medicine, Korea. nuyikim@catholic.ac.kr, iho@catholic.ac.kr
- KMID: 2137844
- DOI: http://doi.org/10.5124/jkma.2005.48.10.993
Abstract
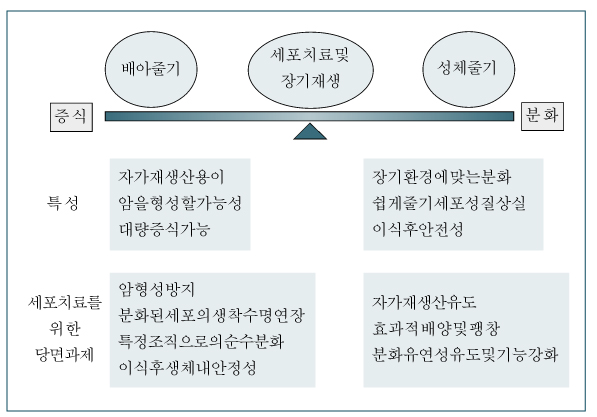
- Adult stem cells and embryonic stem cells are two counterparts of stem cells that can be used for the regeneration of organs and for cell therapy. While each stem cell has its own characteristics, recent findings on the plasticity of adult stem cells are expanding the horizons for cell therapy using these stem cells. In addition, adult stem cells are less prone to the transformation process or inappropriate ectopic differentiation. These characteristics of adult stem cells make them an attractive source of cell therapy and thus being actively exploited for their possible use in cell therapeutic treatment of many intractable diseases. However, many questions remain for the nature or mechanisms of plasticity in adult stem cells, and the task of inducing self-renewal for adult stem cell expansion and engineering has not been accomplished yet. Thus, for successful application of adult stem cells for cell therapy, further understanding on the nature of adult stem cells is necessary, which is critical for the development of high-performance cell therapy and to overcome current limitations in adult stem cell therapies.
MeSH Terms
Figure
Cited by 1 articles
-
Stem Cell Properties of Therapeutic Potential
Geom Seog Seo
Korean J Gastroenterol. 2011;58(3):125-132. doi: 10.4166/kjg.2011.58.3.125.
Reference
-
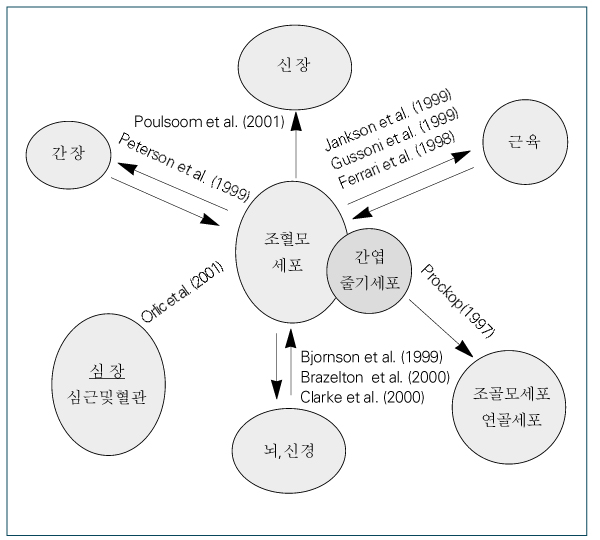
1. Oh IH, Kim DW. Three-dimensional approach to stem cell therapy. J Korean Med Sci. 2002. 17:151–160.
Article3. Kawada H, Ogawa M. Bone marrow origin of hematopoietic progenitors and stem cells in murine muscle. Blood. 2001. 98:2008–2013.
Article4. Lagasse E, Shizuru JA, Uchida N, Tsukamoto A, Weissman IL. Toward regenerative medicine. Immunity. 2001. 14:425–436.
Article5. Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34(-) Lin (-) and CD34(+) Lin (-) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000. 95:2813–2820.
Article6. Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function. Cell. 2001. 105:829–841.7. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.
Article8. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Hedrick MH, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article9. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992. 255:1707–1710.
Article10. Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Reynolds BA, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996. 16:7599–7609.
Article11. Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999. 96:25–34.
Article12. Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001. 412:736–739.
Article13. Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, O'Neil JJ, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000. 97:7999–8004.14. Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development. 1993. 118:33–46.
Article15. Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000. 6:278–282.
Article16. Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004. 84:607–617.
Article17. Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001. 2:75–82.
Article18. Lansdorp PM. Self-renewal of stem cells. Biol Blood Marrow Transplant. 1997. 3:171–178.
Article19. Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002. 109:39–45.
Article20. Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Reya T, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005. 6:314–322.
Article21. Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003. 423:255–260.
Article22. Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Frisen J, et al. Generalized potential of adult neural stem cells. Science. 2000. 288:1660–1663.
Article23. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Sharkis SJ, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001. 105:369–377.
Article24. Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Grompe M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000. 6:1229–1234.
Article25. Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Scott EW, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002. 416:542–545.
Article26. Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002. 416:545–548.
Article27. Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004. 6:532–539.
Article28. Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Wright NA, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000. 406:257.
Article29. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Anversa P, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.
Article30. Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Anversa P, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001. 98:10344–10349.
Article31. Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000. 290:1775–1779.
Article32. Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000. 290:1779–1782.
Article33. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.
Article34. Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Olson L, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002. 99:2199–2204.
Article35. Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001. 56:1666–1672.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Properties of Therapeutic Potential
- Developmental Strategy for Stem Cell Therapy Products in Regulatory Considerations
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- Delivering Factors for Reprogramming a Somatic Cell to Pluripotency
- Stem cells: general information and perspectives