J Vet Sci.
2017 Mar;18(1):1-9. 10.4142/jvs.2017.18.1.1.
Reactive oxygen species-mediated unfolded protein response pathways in preimplantation embryos
- Affiliations
-
- 1Laboratory of Animal Genetic Breeding and Reproduction, Agriculture College of Yanbian University, Yanji 133002, China. nzfang@ybu.edu.cn
- 2National Animal Transmissible Spongiform Encephalopathy Laboratory, Key Laboratory of Animal Epidemiology and Zoonosis of Ministry of Agriculture, College of Veterinary Medicine and State Key Laboratory of Agro Biotechnology, China Agricultural University, Beijing 100193, China.
- KMID: 2412581
- DOI: http://doi.org/10.4142/jvs.2017.18.1.1
Abstract
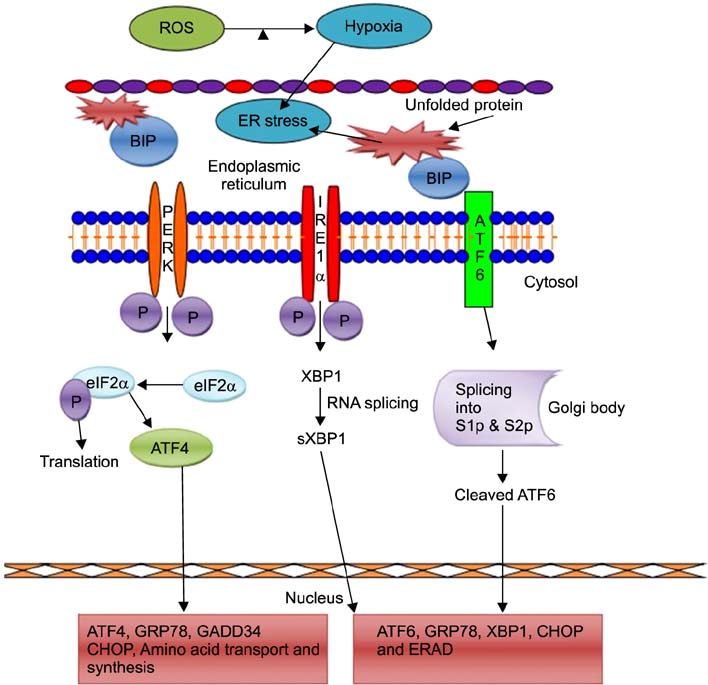
- Excessive production of reactive oxygen species (ROS) and endoplasmic reticulum (ER) stress-mediated responses are critical to embryonic development in the challenging in vitro environment. ROS production increases during early embryonic development with the increase in protein requirements for cell survival and growth. The ER is a multifunctional cellular organelle responsible for protein folding, modification, and cellular homeostasis. ER stress is activated by a variety of factors including ROS. Such stress leads to activation of the adaptive unfolded protein response (UPR), which restores homeostasis. However, chronic stress can exceed the toleration level of the ER, resulting in cellular apoptosis. In this review, we briefly describe the generation and impact of ROS in preimplantation embryo development, the ROS-mediated activation mechanism of the UPR via the ER, and the subsequent activation of signaling pathways following ER stress in preimplantation embryos.
MeSH Terms
Figure
Reference
-
1. Abraham T, Pin CL, Watson AJ. Embryo collection induced transient activation of XBP1 arm of the ER stress response while embryo vitrification does not. Mol Hum Reprod. 2012; 18:229–242.
Article2. Alexiou M, Leese HJ. Purine utilization, de novo synthesis and degradation in mouse preimplantation embryos. Development. 1992; 114:185–192.
Article3. Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirement for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003; 59:939–949.
Article4. Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012; 81:767–793.
Article5. Bain NT, Madan P, Betts DH. The early embryo response to intracellular reactive oxygen species is developmentally regulated. Reprod Fertil Dev. 2011; 23:561–575.
Article6. Basar M, Bozkurt I, Guzeloglu-Kayisli O, Sozen B, Tekmen I, Schatz F, Arici A, Lockwood CJ, Kayisli UA. Unfolded protein response prevents blastocyst formation during preimplantation embryo development in vitro. Fertil Steril. 2014; 102:1777–1784.
Article7. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000; 2:326–332.
Article8. Breckenridge DG, Germain M, Mathani JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003; 22:8608–8618.
Article9. Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AFG, Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013; 301:215–290.10. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum loaded to secretory capacity by processing the XBP-1 mRNA. Nature. 2002; 415:92–96.
Article11. Cebrian-Serrano A, Salvador I, Raga E, Dinnyes A, Silvestre MA. Beneficial effect of melatonin on blastocyst in vitro production from heat-stressed bovine oocytes. Reprod Domest Anim. 2013; 48:738–746.
Article12. Chakrabarti A, Chen AW, Varner JD. A review of the mammalian unfolded protein response. Biotechnol Bioeng. 2011; 108:2777–2793.
Article13. Chason RJ, Csokmay J, Segars JH, DeCherney AH, Armant DR. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol Metab. 2011; 22:412–420.
Article14. Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem. 2002; 277:13045–13052.
Article15. Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003; 23:7198–7209.
Article16. Díaz-Villanueva JF, Díaz-Molina R, García-González V. Protein folding and mechanisms of proteostasis. Int J Mol Sci. 2015; 16:17193–17230.
Article17. Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003; 4:181–191.
Article18. Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999; 286:1882–1888.
Article19. Fischer B, Bavister BD. Oxygen tension in oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993; 99:673–679.
Article20. Gardiner CS, Reed DJ. Synthesis of glutathione in the preimplantation mouse embryo. Arch Biochem Biophys. 1995; 318:30–36.
Article21. Gaut JR, Hendershot LM. The modification and assembly of proteins in the endoplasmic reticulum. Curr Opin Cell Biol. 1993; 5:589–595.
Article22. Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med. 1993; 15:69–75.
Article23. Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 2013; 75:49–67.
Article24. Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surrounding. Hum Reprod Update. 2001; 7:175–189.25. Hall V, Hinichs K, Lazzari G, Betts DH, Hyttel P. Early embryonic development, assisted reproductive technologies, and pluripotent stem cell biology in domestic mammals. Vet J. 2013; 197:128–142.
Article26. Halliwell B. Antioxidant defense mechanisms: from the beginning to the end (of the beginning). Free Radic Res. 1999; 31:261–272.
Article27. Halliwell B, Gutteridge JMC. The chemistry of oxygen radicals and other oxygen-derived species. In : Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. 2nd ed. Oxford: Clarendon Press;1989. p. 22–85.28. Hao L, Vassena R, Wu G, Han Z, Cheng Y, Latham KE, Sapienza C. The unfolded protein response contributes to preimplantation mouse embryo death in the DDK syndrome. Biol Reprod. 2009; 80:944–953.
Article29. Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002; 18:575–599.
Article30. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000; 5:897–904.
Article31. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003; 11:619–633.
Article32. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13:89–102.
Article33. Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996; 44:476–485.
Article34. Hubbard SC, Ivatt RJ. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981; 50:555–583.
Article35. Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994; 16:31–38.
Article36. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000; 92:1564–1572.
Article37. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002; 110:1389–1398.
Article38. King LS, Berg M, Chevalier M, Carey A, Elguindi EC, Blood SY. Isolation, expression and characterization of fully functional nontoxic BiP/GRP78 mutants. Protein Expr Purif. 2001; 22:148–158.
Article39. Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004; 16:343–349.
Article40. Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985; 54:631–664.
Article41. Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev. 2005; 17:371–378.
Article42. Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod. 2002; 17:2686–2693.
Article43. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003; 23:7448–7459.
Article44. Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002; 16:452–466.
Article45. Liu CY, Schröder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinase IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000; 275:24881–24885.
Article46. Lopes AS, Lane M, Thompson JG. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum Reprod. 2010; 25:2762–2773.
Article47. Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006; 26:5688–5697.
Article48. Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002; 318:1351–1365.
Article49. Maity P, Bindu S, Dey S, Goyal M, Alam A, Pal C, Reiter R, Bandyopadhyay U. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J Pineal Res. 2009; 46:314–323.
Article50. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007; 18:716–731.
Article51. Manes C, Lai NC. Nonmitochondrial oxygen utilization by rabbit blastocysts and surface production of superoxide radicals. J Reprod Fertil. 1995; 104:69–75.
Article52. Michalak M, Gye MC. Endoplasmic reticulum stress in preiimplantation embryos. Clin Exp Reprod Med. 2015; 42:1–7.
Article53. Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010; 48:1105–1110.
Article54. Mori K, Ma W, Gething MJ, Sambrook JA. A transmembrane protein with a cdc2+/CDC 28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993; 74:743–756.
Article55. Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T. mRNA splicing-mediated C- terminal replacement of transcription factor Hac1p is required for efficient activation of the unfolded protein response. Proc Natl Acad Sci U S A. 2000; 97:4660–4665.
Article56. Nakayama T, Noda Y, Goto Y, Mori T. Effects of visible light and other environmental factors on the production of oxygen radicals by hamster embryos. Theriogenology. 1994; 41:499–510.
Article57. Nasr-Esfahani MM, Aitken JR, Johnson MH. Hydrogen perioxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990; 109:501–507.
Article58. Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991; 113:551–560.
Article59. Niwa M, Sidrauski C, Kaufman RJ, Walter P. A role of presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell. 1999; 99:691–702.
Article60. Ohlweiler LU, Burm DS, Leivas FG, Moyses AB, Ramos RS, Klein N, Mezzalira JC, Mezzalira A. Intracytoplasmic sperm injection improves in vitro embryo production from poor quality bovine oocytes. Theriogenology. 2013; 79:778–783.
Article61. Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002; 366:585–594.
Article62. Ou XH, Li S, Wang ZB, Li M, Quan S, Xing F, Guo L, Chao SB, Chen Z, Liang XW, Hou Y, Schatten H, Sun QY. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod. 2012; 27:2130–2145.
Article63. Overstrom EW, Duby RT, Dobrinsky J, Roche JF, Boland MP. Viability and oxidative metabolism of the bovine blastocyst. Theriogenology. 1992; 37:269.
Article64. Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003; 18:2270–2274.
Article65. Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001; 13:349–355.
Article66. Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006; 22:257–273.
Article67. Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, Horton HF, Scott A, Orkin SH, Byrne MC, Grusby MJ, Glimcher LH. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000; 14:152–157.
Article68. Reverendo M, Soares AR, Pereira PM, Carreto L, Ferreira M, Gatti E, Pierre P, Moura GR, Santos MA. TRNA mutations that effect decoding fidelity deregulate development and the proteostasis network in zebrafish. RNA Biol. 2014; 11:1199–1213.
Article69. Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002; 110:1383–1388.
Article70. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.
Article71. Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001; 7:1165–1176.
Article72. Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005; 569:29–63.
Article73. Shah SZ, Zhao D, Khan SH, Yang L. Unfolded protein response pathways in neurodegenerative diseases. J Mol Neurosci. 2015; 57:529–637.
Article74. Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002; 3:99–111.
Article75. Succu S, Pasciu V, Manca ME, Celucci S, Torres-Rovira L, Leoni GG, Zinellu A, Carru C, Naitana S, Berlinguer F. Dose-dependent effect of melatonin on postwarming development of vitrified ovine embryos. Theriogenology. 2014; 81:1058–1066.
Article76. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011; 13:184–190.
Article77. Tatone C, Di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecol Endocrinol. 2010; 26:563–567.
Article78. Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000; 118:47–55.
Article79. Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil. 1996; 106:299–306.
Article80. Thuerauf DJ, Morrison L, Glembotski CC. Opposing roles for ATF6α and ATF6β in endoplasmic reticulum stress response gene induction. J Biol Chem. 2004; 279:21078–21084.
Article81. Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998; 12:1812–1824.
Article82. Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998; 17:5708–5717.
Article83. Wu LL, Russel DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocytes complexes impairs pentraxin-3 sec secretion, mitochondrial membrane potential (ΔΨm), and embryo development. Mol Endocrinol. 2012; 26:562–573.
Article84. Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev Cell. 2007; 13:365–376.
Article85. Yoon SB, Choi SA, Sim BW, Kim JS, Mun SE, Jeong PS, Yang HJ, Lee Y, Park YH, Song BS, Kim YH, Jeong KJ, Huh JW, Lee SR, Kim SU, Chang KT. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol Reprod. 2014; 90:104.86. Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998; 273:33741–33749.
Article87. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001; 107:881–891.
Article88. Yoshida H, Nadanaka S, Sato R, Mori K. XBP1 is critical to protect cells from endoplasmic reticulum stress: evidence from site-2 protease-deficient Chinese hamster ovary cells. Cell Struct Funct. 2006; 31:117–125.
Article89. Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000; 20:6755–6767.
Article90. Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factor 6α and 6β that activates the mammalian unfolded protein response. Mol Cell Biol. 2001; 21:1239–1248.
Article91. Yuan YQ, Van Soom A, Coopman FOJ, Mintiens K, Boerjan ML, Van Zeveren A, de Kruif A, Peelman LJ. Influence of oxygen tension on apoptosis and hatching in bovine embryos cultured in vitro. Theriogenology. 2003; 59:1585–1596.
Article92. Zhao HW, Haddad GG. Review: Hypoxic and oxidative stress resistance in Drosophila melanogaster. Placenta. 2011; 32:Suppl 2. S104–S108.93. Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol. 2005; 283:40–57.
Article94. Zhang JY, Diao YF, Kim HR, Jin DI. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLos One. 2012; 7:e40433.
Article95. Zhang JY, Diao YF, Oqani RK, Han RX, Jin DI. Effect of endoplasmic reticulum stress on porcine oocytes maturation and parthenogenetic embryonic development in vitro. Biol Reprod. 2012; 86:128.96. Zimmer T, Ogura A, Ohta A, Takagi M. Misfolded membrane-bound cytochrome P450 activates KAR2 induction through two distinct mechanisms. J Biochem. 1999; 126:1080–1089.
Article97. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998; 12:982–995.
Article