J Vet Sci.
2017 Jun;18(2):129-140. 10.4142/jvs.2017.18.2.129.
Apoptosis in response to heat stress is positively associated with heat-shock protein 90 expression in chicken myocardial cells in vitro
- Affiliations
-
- 1College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China. b_endong@njau.edu.cn
- 2College of Animal Science and Technology, Jinling Institute of Technology, Nanjing 210038, China.
- 3Institute for Animal Hygiene, Animal Welfare and Farm Animal Behaviour, University of Veterinary Medicine Hannover, Foundation, Hannover 30173, Germany.
- KMID: 2412565
- DOI: http://doi.org/10.4142/jvs.2017.18.2.129
Abstract
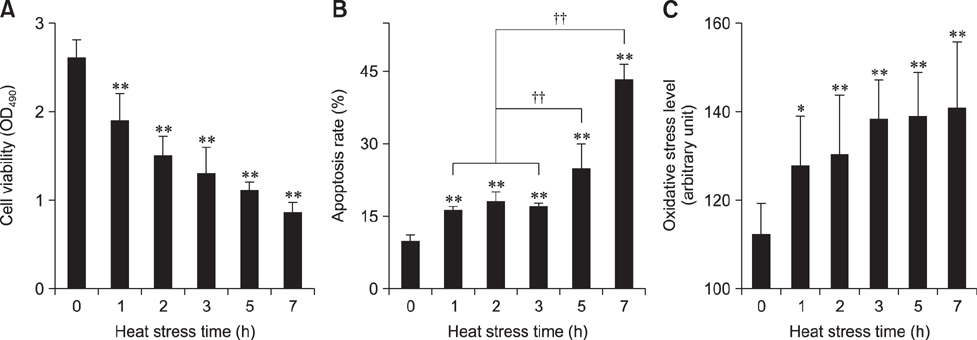
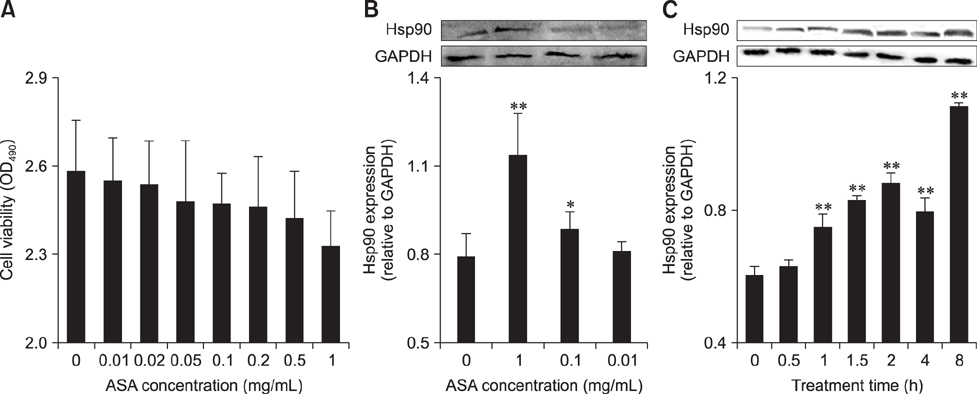
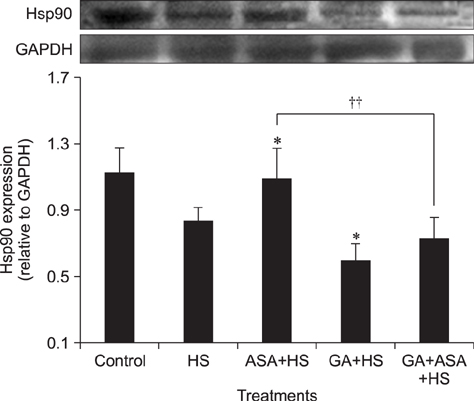
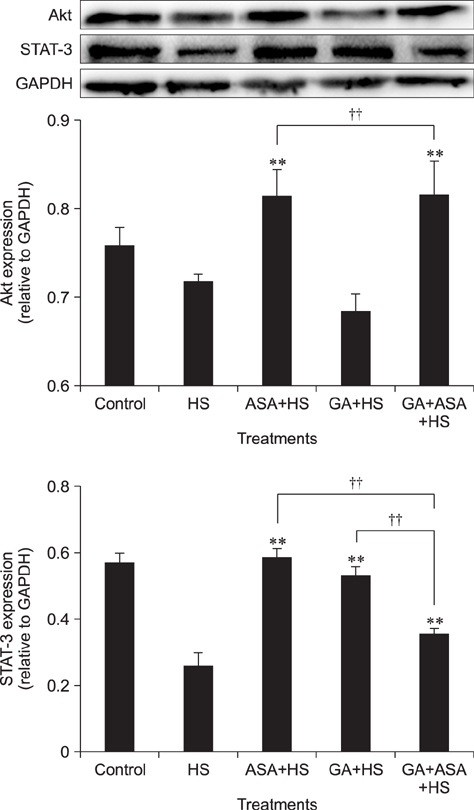
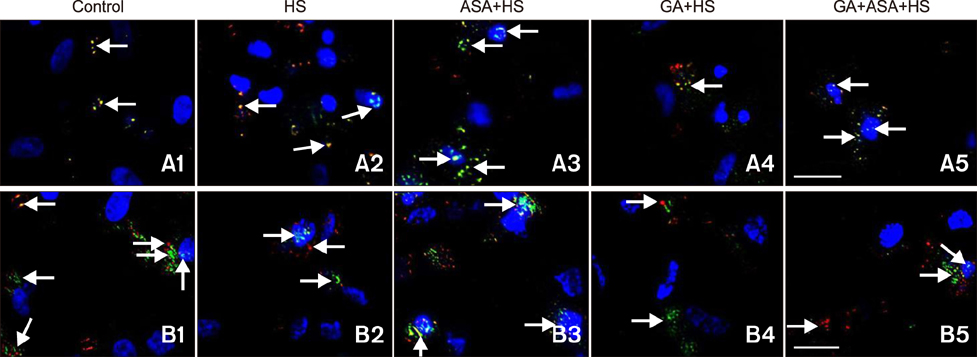
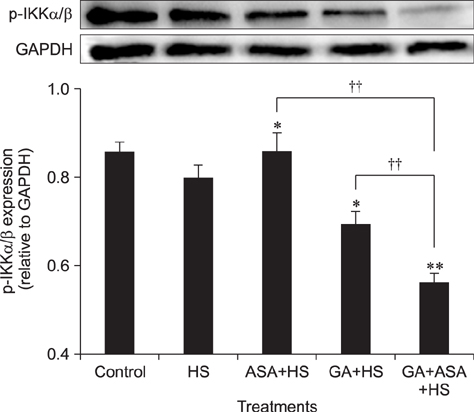
- To determine heat-shock protein (Hsp)90 expression is connected with cellular apoptotic response to heat stress and its mechanism, chicken (Gallus gallus) primary myocardial cells were treated with the Hsp90 promoter, aspirin, and its inhibitor, geldanamycin (GA), before heat stress. Cellular viability, heat-stressed apoptosis and reactive oxygen species level under different treatments were measured, and the expression of key proteins of the signaling pathway related to Hsp90 and their colocalization with Hsp90 were detected. The results showed that aspirin treatment increased the expression of protein kinase B (Akt), the signal transducer and activator of transcription (STAT)-3 and p-IKKα/β and the colocalization of Akt and STAT-3 with Hsp90 during heat stress, which was accompanied by improved viability and low apoptosis. GA significantly inhibited Akt expression and p-IKKα/β level, but not STAT-3 quantity, while the colocalization of Akt and STAT-3 with Hsp90 was weakened, followed by lower cell viability and higher apoptosis. Aspirin after GA treatment partially improved the stress response and apoptosis rate of tested cells caused by the recovery of Akt expression and colocalization, rather than the level of STAT-3 (including its co-localization with Hsp90) and p-IKKα/β. Therefore, Hsp90 expression has a positive effect on cellular capacity to resist heat-stressed injury and apoptosis. Moreover, inhibition of Hsp90 before stress partially attenuated its positive effects.
Keyword
MeSH Terms
-
Animals
Apoptosis/*physiology
Aspirin/pharmacology
Benzoquinones/pharmacology
Blotting, Western/veterinary
Chick Embryo/cytology
Flow Cytometry/veterinary
HSP90 Heat-Shock Proteins/agonists/antagonists & inhibitors/*metabolism/physiology
Heat-Shock Response/*physiology
In Vitro Techniques
Lactams, Macrocyclic/pharmacology
Myocardium/cytology/*metabolism
Reactive Oxygen Species/metabolism
Benzoquinones
HSP90 Heat-Shock Proteins
Lactams, Macrocyclic
Reactive Oxygen Species
Aspirin
Figure
Reference
-
1. Amici C, Rossi A, Santoro GM. Aspirin enhances thermotolerance in human erythroleukemic cells: an effect associated with the modulation of the heat response of the heat shock responce. Cancer Res. 1995; 55:4452–4457.2. Bagatell R, Beliakoff J, David CL, Marron MT, Whitesell L. Hsp90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumor cells and demonstrate synergistic anticancer activity with cisplatin. Int J Cancer. 2005; 113:179–188.
Article3. Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene. 2004; 23:5378–5386.
Article4. Chaudhury S, Welch TR, Blagg BSJ. Hsp90 as a target for drug development. ChemMedChem. 2006; 1:1331–1340.
Article5. Chávez-Mardones J, Gallardo-Escárate C. Immune response of apoptosis-related cysteine peptidases from the red abalone Haliotis rufescens (HrCas8 and HrCas3): molecular characterization and transcription expression. Fish Shellfish Immunol. 2014; 39:90–98.
Article6. Clark CB, Rane MJ, El Mehdi D, Miller CJ, Sachleben LR Jr, Gozal E. Role of oxidative stress in geldanamycin-induced cytotoxicity and disruption of Hsp90 signaling complex. Free Radic Biol Med. 2009; 47:1440–1449.
Article7. Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, Gonzalez-Gallego J. Quercetin decreases oxidative stress, NF-κB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005; 135:2299–2304.
Article8. Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 2003; 278:28490–28500.
Article9. Faissner A, Heck N, Dobbertin A, Garwood J. DSD-1-proteoglycan/phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues. Adv Exp Med Biol. 2006; 557:25–53.
Article10. Feng J, Zhang M, Zheng S, Xie P, Ma A. Effects of high temperature on multiple parameters of broilers in vitro and in vivo. Poult Sci. 2008; 87:2133–2139.
Article11. Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, Campos L. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008; 13:357–364.
Article12. Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002; 90:866–873.
Article13. Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006; 25:4798–4811.
Article14. Giardina C, Lis JT. Sodium salicylate and yeast heat shock gene transcription. J Biol Chem. 1995; 270:10369–10372.
Article15. Gu XH, Hao Y, Wang XL. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers. 2. Intestinal oxidative stress. Poult Sci. 2012; 91:790–799.
Article16. Hao Y, Gu XH. Effects of heat shock protein 90 expression on pectoralis major oxidation in broilers exposed to acute heat stress. Poult Sci. 2014; 93:2709–2717.
Article17. Hatada EN, Krappmann D, Scheidereit C. NF-κB and the innate immune response. Curr Opin Immunol. 2000; 12:52–58.18. Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J. 2002; 16:114–116.
Article19. Islam A, Lv YJ, Abdelnasir A, Rehana B, Liu ZJ, Zhang M, Tang S, Cheng YF, Chen HB, Hartung J, Bao ED. The role of Hsp90α in heat-induced apoptosis and cell damage in primary myocardial cell cultures of neonatal rats. Genet Mol Res. 2013; 12:6080–6091.
Article20. Jin M, Otaka M, Okuyama A, Itoh S, Otani S, Odashima M, Iwabuchi A, Konishi N, Wada I, Pacheco I, Itoh H, Tashima Y, Masamune O, Watanabe S. Association of 72-kDa heat shock protein expression with adaptation to aspirin in rat gastric mucosa. Dig Dis Sci. 1999; 44:1401–1407.21. Jurivich DA, Pachetti C, Qiu L, Welk JF. Salicylate triggers heat shock factor differently than heat. J Biol Chem. 1995; 270:24489–24495.
Article22. Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C. Characterization of the signal that directs Bcl-xL, but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003; 160:53–64.
Article23. Koo HN, Oh SY, Kang KI, Moon DY, Kim HD, Kang HS. Modulation of HSP70 and HSP90 expression by sodium salicylate and aspirin in fish cell line CHSE-214. Zoolog Sci. 2000; 17:1275–1282.
Article24. Lee KH, Jang Y, Chung JH. Heat shock protein 90 regulates IκB kinase complex and NF-κB activation in angiotensin II-induced cardiac cell hypertrophy. Exp Mol Med. 2010; 42:703–711.
Article25. Liu CM, Zheng G, Ming QL, Chao C, Sun JM. Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. J Agric Food Chem. 2013; 61:1146–1154.
Article26. Mohan S, Konopinski R, Yan B, Centonze VE, Natarajan M. High glucose-induced IKK-Hsp-90 interaction contributes to endothelial dysfunction. Am J Physiol Cell Physiol. 2009; 296:C182–C192.
Article27. Mujahid A, Pumford NR, Bottje W, Nakagawa K, Miyazawa T, Akiba Y, Toyomizu M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. Poult Sci. 2007; 44:439–445.
Article28. Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007; 32:517–530.
Article29. Padmini E, Usha Rani RM. Heat-shock protein 90 alpha (HSP90α) modulates signaling pathways towards tolerance of oxidative stress and enhanced survival of hepatocytes of Mugil cephalus. Cell Stress Chaperones. 2011; 16:411–425.
Article30. Shimp SK 3rd, Parson CD, Regna NL, Thomas AN, Chafin CB, Reilly CM, Nichole Rylander M. HSP90 inhibition by 17-DMAG reduces inflammation in J774 macrophages through suppression of Akt and nuclear factor- κB pathways. Inflamm Res. 2012; 61:521–533.
Article31. Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000; 97:10832–10837.
Article32. Schoof N, von Bonin F, Trümper L, Kube D. HSP90 is essential for Jak-STAT signaling in classical Hodgkin lymphoma cells. Cell Commun Signal. 2009; 7:17.
Article33. Shang L, Tomasi TB. The heat shock protein 90-CDC37 chaperone complex is required for signaling by types I and II interferons. J Biol Chem. 2006; 281:1876–1884.
Article34. Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005; 280:39962–39969.
Article35. Wu T, Mohan C. The AKT axis as a therapeutic target in autoimmune diseases. Endocr Metab Immune Disord Drug Targets. 2009; 9:145–150.
Article36. Zhang H, Chung D, Yang YC, Neely L, Tsurumoto S, Fan J, Zhang L, Biamonte M, Brekken J, Lundgren K, Burrows F. Identification of new biomarkers for clinical trials of Hsp90 inhibitors. Mol Cancer Ther. 2006; 5:1256–1264.
Article37. Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. HSP90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005; 24:3954–3963.
Article38. Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol Cell. 1998; 2:101–108.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- Expression of Heat Shock Protein 70 in Vein Endothelial Cells Induced by Shear Stress
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell