J Vet Sci.
2017 Aug;18(S1):315-322. 10.4142/jvs.2017.18.S1.315.
Influence of vaccine potency and booster administration of foot-and-mouth disease vaccines on the antibody response in calves with maternal antibodies
- Affiliations
-
- 1Institute of Foot and Mouth Disease (SAP), Ministry of Food, Agriculture and Livestock, Ankara 06044, Turkey. cancokcaliskan@gmail.com
- 2Directorate-General for Agriculture and Rural Development, Ministry of Food, Agriculture and Livestock, Ankara 06044, Turkey.
- 3Directorate-General for State Farms, Ministry of Food, Agriculture and Livestock, Ankara 06044, Turkey.
- KMID: 2412556
- DOI: http://doi.org/10.4142/jvs.2017.18.S1.315
Abstract
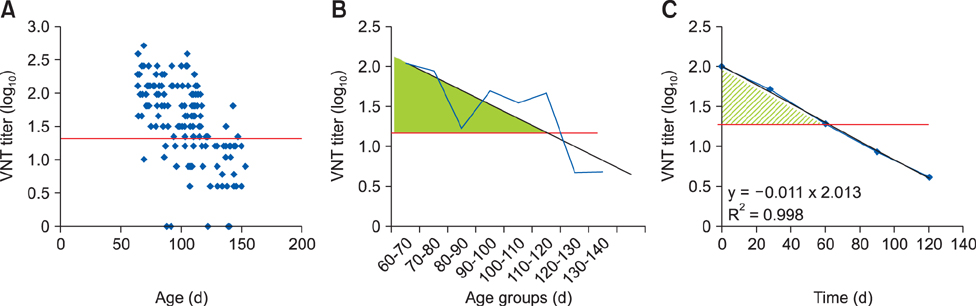
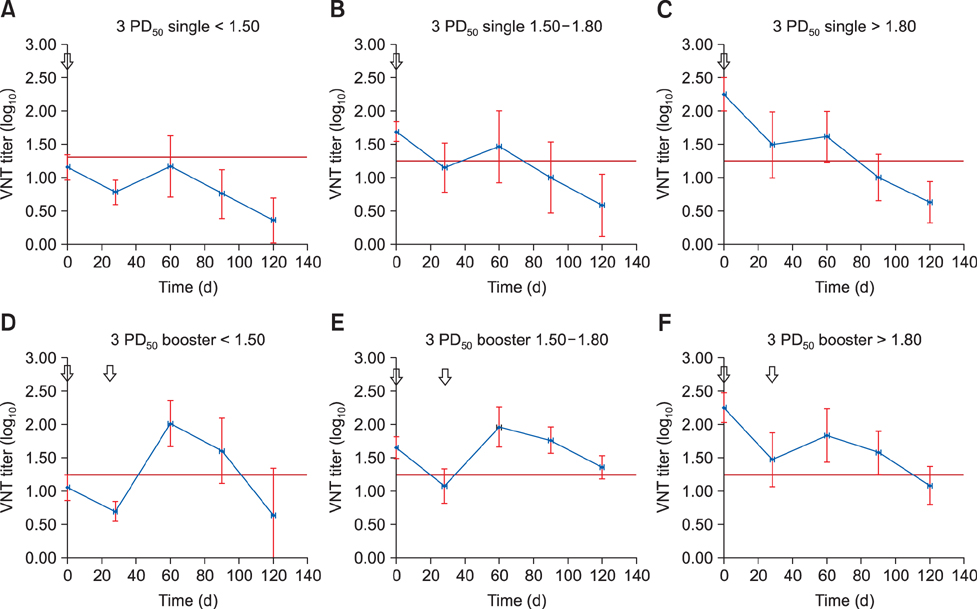
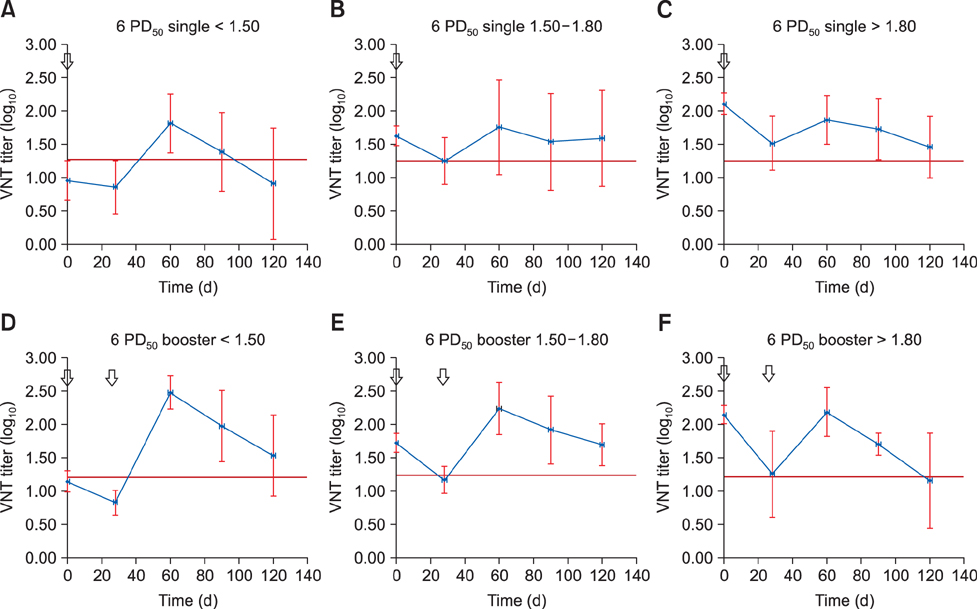
- Foot-and-mouth disease is one of the most important viral diseases of cloven-hoofed animals. Mass vaccination is an effective method to control the disease and is frequently utilized in endemic regions. Sufficient protection of young animals is important in mass vaccination campaigns. Maternal antibodies negatively affect the success of vaccination. Hence, determination of the optimal vaccination age is crucial for the uninterrupted protection of young animals. This study was performed to identify the effect of vaccine potency and booster administration on serum neutralizing antibody titers of calves with different levels of maternal antibodies. Calves (n = 111) on a state farm were used in this study. Oil adjuvant foot-and-mouth disease vaccines with 3 PDâ‚…â‚€ and 6 PDâ‚…â‚€ potencies were used with or without booster administration. Serum samples were collected each month up to day 120 postvaccination. Virus neutralization tests were used to measure the serum neutralizing antibody titers and estimate the protection period by using pre-determined cut-off values for protection. The results revealed that a vaccination with a 6 PDâ‚…â‚€ potency vaccine, preferably followed by a booster dose, should be used to overcome maternal immunity for incessant protection.
MeSH Terms
-
Animals
Antibody Formation/immunology
Cattle
Cattle Diseases/immunology/*prevention & control/virology
Foot-and-Mouth Disease/immunology/*prevention & control
*Foot-and-Mouth Disease Virus/immunology
Neutralization Tests/veterinary
Viral Vaccines/administration & dosage/immunology/*therapeutic use
Viral Vaccines
Figure
Reference
-
1. Bielefeldt-Ohmann H, Prow NA, Wang W, Tan CS, Coyle M, Douma A, Hobson-Peters J, Kidd L, Hall RA, Petrovsky N. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Vet Res. 2014; 45:130.
Article2. Bucafusco D, Di Giacomo S, Pega J, Juncos MS, Schammas JM, Pérez-Filgueira M, Capozzo AV. Influence of antibodies transferred by colostrum in the immune responses of calves to current foot-and-mouth disease vaccines. Vaccine. 2014; 32:6576–6582.
Article3. Chase CC, Hurley DJ, Reber AJ. Neonatal immune development in the calf and its impact on vaccine response. Vet Clin North Am Food Anim Pract. 2008; 24:87–104.
Article4. Council of Europe, European Pharmacopoeia Commission, European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia. 8th ed. Strasbourg: Council of Europe, European Directorate for the Quality of Medicines and Healthcare;2013.5. Davis EG, Bello NM, Bryan AJ, Hankins K, Wilkerson M. Characterisation of immune responses in healthy foals when a multivalent vaccine protocol was initiated at age 90 or 180 days. Equine Vet J. 2015; 47:667–674.
Article6. Dekker A, Eblé P, Stockhofe N, Chénard G. Intratypic heterologous vaccination of calves can induce an antibody response in presence of maternal antibodies against foot-and-mouth disease virus. BMC Vet Res. 2014; 10:127.
Article7. Downey ED, Tait RG Jr, Mayes MS, Garrick DJ, Ridpath J, Reecy JM. Effects of calf age and dam age on circulating BVDV II antibody levels prior to vaccination in Angus weanling calves. Reports No. 214. Ames: Iowa State Research Farm Progress Reports (US);2011.8. Ellis J, Gow S, Bolton M, Burdett W, Nordstrom S. Inhibition of priming for bovine respiratory syncytial virus-specific protective immune responses following parenteral vaccination of passively immune calves. Can Vet J. 2014; 55:1180–1185.9. Elnekave E, Zamir L, Hamd F, Even Tov B, Klement E. Risk factors for foot and mouth disease outbreaks in grazing beef cattle herds. Prev Vet Med. 2015; 120:236–240.
Article10. Endsley JJ, Roth JA, Ridpath J, Neill J. Maternal antibody blocks humoral but not T cell responses to BVDV. Biologicals. 2003; 31:123–125.
Article11. Foote MR, Nonnecke BJ, Beitz DC, Waters WR. Antigen-specific B-cell responses by neonatal calves after early vaccination. J Dairy Sci. 2007; 90:5208–5217.
Article12. Guzman E, Taylor G, Charleston B, Ellis SA. Induction of a cross-reactive CD8+ T cell response following foot-and-mouth disease virus vaccination. J Virol. 2010; 84:12375–12384.
Article13. Hodgins DC, Shewen PE, Dewey CE. Influence of age and maternal antibodies on antibody responses of neonatal piglets vaccinated against Mycoplasma hyopneumoniae. J Swine Health Prod. 2004; 12:10–16.14. Jamal SM, Bouma A, van den Broek J, Stegeman A, Chénard G, Dekker A. Foot-and-mouth disease vaccine potency testing: the influence of serotype, type of adjuvant, valency, fractionation method, and virus culture on the dose-response curve in cattle. Vaccine. 2008; 26:6317–6321.
Article15. Knight-Jones TJ, Bulut AN, Gubbins S, Stärk KD, Pfeiffer DU, Sumption KJ, Paton DJ. Retrospective evaluation of foot-and-mouth disease vaccine effectiveness in Turkey. Vaccine. 2014; 32:1848–1855.
Article16. Knight-Jones TJ, Gubbins S, Bulut AN, Stärk KD, Pfeiffer DU, Sumption KJ, Paton DJ. Mass vaccination, immunity and coverage: modelling population protection against foot-and-mouth disease in Turkish cattle. Sci Rep. 2016; 6:22121.
Article17. Lee HS, Lee NH, Seo MG, Ko YJ, Kim B, Lee JB, Kim JS, Park S, Shin YK. Serological responses after vaccination of growing pigs with foot-and-mouth disease trivalent (type O, A and Asia1) vaccine. Vet Microbiol. 2013; 164:239–245.
Article18. Murphy JM, Hagey JV, Chigerwe M. Comparison of serum immunoglobulin G half-life in dairy calves fed colostrum, colostrum replacer or administered with intravenous bovine plasma. Vet Immunol Immunopathol. 2014; 158:233–237.
Article19. Nicholls MJ, Black L, Rweyemamu MM, Genovese J, Ferrari R, Hammant CA, de Silva E, Umehara O. The effect of maternally derived antibodies on the response of calves to vaccination against foot and mouth disease. J Hyg (Lond). 1984; 92:105–116.
Article20. Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol. 2014; 5:446.
Article21. Patil PK, Sajjanar CM, Natarajan C, Bayry J. Neutralizing antibody responses to foot-and-mouth disease quadrivalent (type O, A, C and Asia 1) vaccines in growing calves with pre-existing maternal antibodies. Vet Microbiol. 2014; 169:233–235.
Article22. Polewicz M, Gracia A, Buchanan R, Strom S, Halperin SA, Potter AA, Babiuk LA, Gerdts V. Influence of maternal antibodies on active pertussis toxoid immunization of neonatal mice and piglets. Vaccine. 2011; 29:7718–7726.
Article23. Sadir AM, Schudel AA, Laporte O, Braun M, Margni RA. Response to foot-and-mouth disease vaccines in newborn calves. Influence of age, colostral antibodies and adjuvants. Epidemiol Infect. 1988; 100:135–144.
Article24. Shankar H, Uppal PK. Immune response of newborn calves to vaccination with foot-and-mouth disease vaccine. Rev Sci Tech Off Int Epiz. 1982; 1:403–414.
Article25. Späth EJ, Smitsaart E, Casaro AP, Fondevila N, Fernández F, Leunda MR, Compaired D, Buffarini M, Pessi H. Immune response of calves to foot-and-mouth disease virus vaccine emulsified with oil adjuvant. Strategies of vaccination. Vaccine. 1995; 13:909–914.
Article26. Vidor E. Vaccination of newborns against hepatitis A in the presence of maternally derived antibodies. J Comp Pathol. 2007; 137:Suppl 1. S42–S45.
Article27. World Organisation for Animal Health (OIE). Foot and mouth disease. OIE. Terrestrial Animal Health Code. 20th ed. Paris: OIE;2011. p. 437–463.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of simultaneous administration of foot-and-mouth disease (FMD) and anthrax vaccines on antibody response to FMD in sheep
- QS-21 enhances the early antibody response to oil adjuvant foot-and-mouth disease vaccine in cattle
- Increased humoral antibody response of foot-and-mouth disease virus vaccine in growing pigs pre-treated with poly-γ-glutamic acid
- Genetic identification and serological evaluation of commercial inactivated foot-and-mouth disease virus vaccine in pigs
- Immune responses in pigs and cattle vaccinated with half-volume foot-and-mouth disease vaccine