J Vet Sci.
2017 Sep;18(3):267-272. 10.4142/jvs.2017.18.3.267.
Inoculation of Lewis lung carcinoma cells enhances formalin-induced pain behavior and spinal Fos expression in mice
- Affiliations
-
- 1Department of Physiology and Medical Science and Brain Research Institute, College of Medicine, Chungnam National University, Daejeon 35015, Korea. kim0827@cnu.ac.kr
- 2KM Fundamental Research Division, Korea Institute of Oriental Medicine, Daejeon 34054, Korea.
- 3Department of Meridian & Acupoint, College of Oriental Medicine, Wonkwang University, Iksan 54538, Korea.
- KMID: 2412443
- DOI: http://doi.org/10.4142/jvs.2017.18.3.267
Abstract
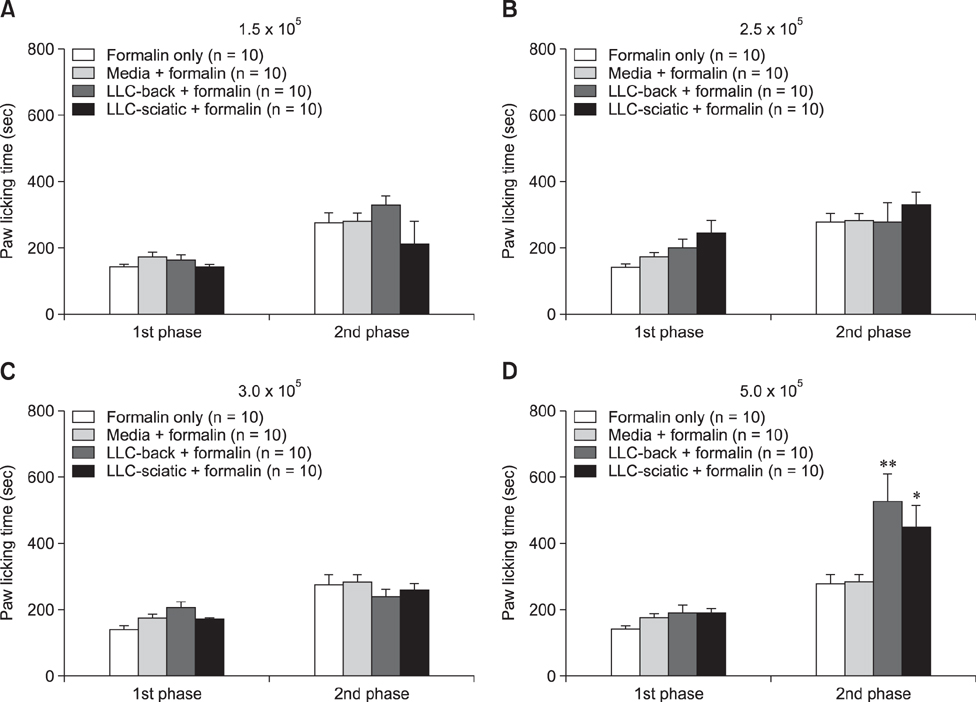
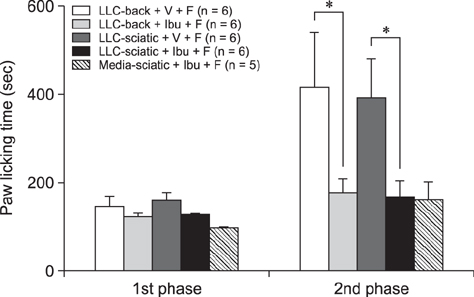
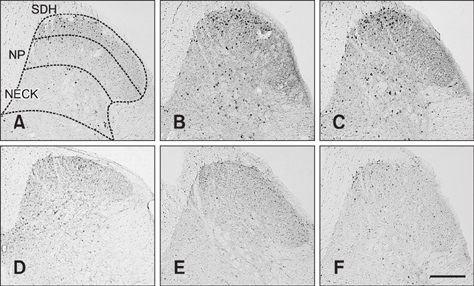
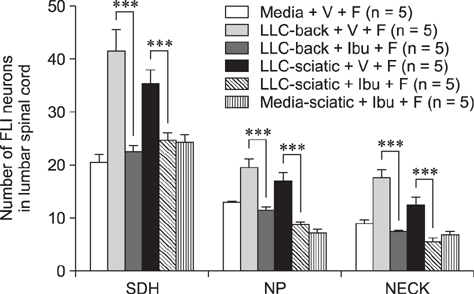
- The incidence of lung cancer has rapidly increased and cancer patients at a later cancer stage frequently suffer from unbearable cancer-associated pain. However, the pathophysiology of lung cancer pain has not been fully described due to a lack of appropriate animal models. This study was designed to determine the effect of Lewis lung carcinoma (LLC) cell inoculation on formalin-induced pain behavior and spinal Fos expression in C57BL/6 mice. LLC cells (1.5 × 10âµ, 2.5 × 10âµ, 3.0 × 10âµ or 5.0 × 10âµ) were inoculated into back or peri-sciatic nerve areas. Back area inoculation was adopted to determine the effect of cancer cell circulating factors and the peri-sciatic nerve area was used to evaluate the possible effects of cancer cell contacting and circulating factors on formalin-induced pain. At postinoculation day 7, LLC cell (5.0 × 10âµ) inoculations in both back and peri-sciatic nerve area significantly increased formalin-induced paw-licking time and spinal Fos expression over those in cell-media-inoculated (control) mice. Enhanced pain behavior and spinal Fos expression were significantly suppressed by ibuprofen pretreatment (250 mg/kg). The results of this study suggest that LLC cell circulating factors and inflammatory responses may be critical in enhancing pain sensation in the early stage of lung cancer cell inoculation.
Keyword
MeSH Terms
-
Analgesics, Non-Narcotic/therapeutic use
Animals
Cancer Pain/drug therapy/*etiology
Carcinoma, Lewis Lung/*complications
Formaldehyde/pharmacology
Ibuprofen/therapeutic use
Male
Mice
Mice, Inbred C57BL
Neoplasm Transplantation
Oncogene Proteins v-fos/*metabolism
Pain/chemically induced/etiology/psychology
Spinal Cord/drug effects/*metabolism
Spinal Cord Dorsal Horn/drug effects/metabolism
Analgesics, Non-Narcotic
Oncogene Proteins v-fos
Formaldehyde
Ibuprofen
Figure
Reference
-
1. Abbadie C, Besson JM. Chronic treatments with aspirin or acetaminophen reduce both the development of polyarthritis and Fos-like immunoreactivity in rat lumbar spinal cord. Pain. 1994; 57:45–54.
Article2. Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995; 60:91–102.
Article3. Bennett GJ. Pathophysiology and animal models of cancer-related painful peripheral neuropathy. Oncologist. 2010; 15(Suppl 2):9–12.
Article4. Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012; 153:359–365.
Article5. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917.
Article6. Hwang HJ, Kim P, Kim CJ, Lee HJ, Shim I, Yin CS, Yang Y, Hahm DH. Antinociceptive effect of amygdalin isolated from Prunus armeniaca on formalin-induced pain in rats. Biol Pharm Bull. 2008; 31:1559–1564.
Article7. Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, Lee JH. Intrathecal treatment with σ1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol. 2006; 148:490–498.
Article8. Kim KW, Kim HW, Li J, Kwon YB. Effect of bee venom acupuncture on methamphetamine-induced hyperactivity, hyperthermia and Fos expression in mice. Brain Res Bull. 2011; 84:61–68.
Article9. Kuraishi Y, Iida Y, Zhang HW, Uehara S, Nojima H, Murata J, Saiki I, Takahata H, Ouchi H. Suppression by gabapentin of pain-related mechano-responses in mice given orthotopic tumor inoculation. Biol Pharm Bull. 2003; 26:550–552.
Article10. Mystakidou K, Parpa E, Tsilika E, Pathiaki M, Galanos A, Vlahos L. Comparison of pain quality descriptors in cancer patients with nociceptive and neuropathic pain. In Vivo. 2007; 21:93–97.11. Pacharinsak C, Beitz A. Animal models of cancer pain. Comp Med. 2008; 58:220–233.12. Roh DH, Yoon SY. Sigma-1 receptor antagonist, BD1047 reduces nociceptive responses and phosphorylation of p38 MAPK in mice orofacial formalin model. Biol Pharm Bull. 2014; 37:145–151.
Article13. Sasamura T, Nakamura S, Iida Y, Fujii H, Murata J, Saiki I, Nojima H, Kuraishi Y. Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol. 2002; 441:185–191.
Article14. Sufka KJ, Watson GS, Nothdurft RE, Mogil JS. Scoring the mouse formalin test: validation study. Eur J Pain. 1998; 2:351–358.
Article15. Wilkie DJ, Huang HY, Reilly N, Cain KC. Nociceptive and neuropathic pain in patients with lung cancer: a comparison of pain quality descriptors. J Pain Symptom Manage. 2001; 22:899–910.16. Yashpal K, Coderre TJ. Influence of formalin concentration on the antinociceptive effects of anti-inflammatory drugs in the formalin test in rats: separate mechanisms underlying the nociceptive effects of low- and high-concentration formalin. Eur J Pain. 1998; 2:63–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preemptive Effect of Ketamine on Inflammatory Pain and Spinal c-fos Expression Induced by Formalin in the Rat
- Effects of Naloxone on Morphine Analgesia and Spinal c-fos Expression in Rat Formalin Test
- The Effects of Pre-emptive Administration of Ketamine and norBNI on Pain Behavior, c-Fos, and Prodynorphin Protein Expression in the Rat Spinal Cord after Formalin-induced Pain Is Modulated by the DREAM Protein
- Effect of Interleukin-12 on the Expression of E-selectin in Mouse Model of Lewis Lung Carcinoma
- Effect of Intrathecal COX Inhibitors on Inflammatory Pain and c-Fos Expression in Central Nervous System Induced by Formalin Injection in Rat